Professional Documents
Culture Documents
Tutorial Problems, 2013/2014: Che 153 Organic Chemistry For Engineers
Tutorial Problems, 2013/2014: Che 153 Organic Chemistry For Engineers
Uploaded by
Ing Rashid BawahOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tutorial Problems, 2013/2014: Che 153 Organic Chemistry For Engineers
Tutorial Problems, 2013/2014: Che 153 Organic Chemistry For Engineers
Uploaded by
Ing Rashid BawahCopyright:
Available Formats
TUTORIAL PROBLEMS, 2013/2014 ChE 153 Organic Chemistry for Engineers
KNUST- CHEMICAL ENGINEERING
Write structural formulas for each of the following: 1-Bromo-6-methylcyclohexene 4-Methyl-4-penten-2-ol (Z)-3-Methyl-2-hexene Vinylcycloheptane 1,1-Diallylcyclopropane trans-1-Isopropenyl-3-methylcyclohexane cis-3-Octene
Write a structural formula or build a
molecular model and give a correct IUPAC
name for each alkene of molecular
formula C7H14 that has a tetrasubstituted
double bond.
Que 3. Write the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a)Hydrogen chloride (b) Hydrogen bromide (c) Hydrogen bromide in the presence of peroxides (d) Hydrogen iodide (e) Dilute sulfuric acid (f ) Diborane in diglyme, followed by basic hydrogen peroxide (g) Bromine in carbon tetrachloride (h) Bromine in water (i ) Peroxyacetic acid (j) Ozone
Que 4. Match the following alkenes with the appropriate heats of hydrogenation: (a) 1-Pentene (b) (E)-4,4-Dimethyl-2-pentene (c) (Z)-4-Methyl-2-pentene (d) (Z)-2,2,5,5-Tetramethyl-3-hexene (e) 2,4-Dimethyl-2-pentene Heats of hydrogenation in kJ/mol (kcal/mol): 151(36.2); 122(29.3); 114(27.3); 111(26.5); 105(25.1).
Que 5. Reaction of 3,3-dimethyl-1-butene with hydrogen iodide yields two compounds A and B, each having the molecular formula C6H13I, in the ratio A:B 90:10. Compound A, on being heated with potassium hydroxide in n-propyl alcohol, gives only 3,3-dimethyl-1-butene. Compound B undergoes elimination under these conditions to give 2,3-dimethyl-2-butene as the major product. Suggest structures for compounds A and B, and write a reasonable mechanism for the formation
Que 6. Specify reagents suitable for converting 3-ethyl-2-pentene to each of the following: (a) 2,3-Dibromo-3-ethylpentane (b) 3-Chloro-3-ethylpentane (c) 2-Bromo-3-ethylpentane (d) 3-Ethyl-3-pentanol (e) 3-Ethyl-2-pentanol (f ) 3-Ethyl-2,3-epoxypentane (g) 3-Ethylpentane
Consider the following alkene:
(a) What is the molecular formula of this alkene? (b) What is its IUPAC name? (c) How many carbon atoms are sp2-hybridized in this alkene? How many are sp3hybridized? (d) How many _ bonds are of the sp2sp3 type? How many are of the sp3sp3 type?
Determine the configuration of each of the following alkenes as Z or E as appropriate:
Write structural formulas or build molecular models and give the IUPAC names for all the alkenes of molecular formula C6H12 that contain a trisubstituted double bond.
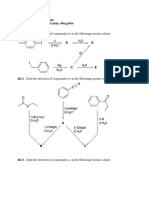
Name the compounds below.
When the alkene 3,3-dimethylbutene is treated with hydrogen iodide, there is obtained a mixture of products; 3-iodo2,2-dimethylbutane and 3-iodo2,3dimethylbutane. Write the eqn. for the reaction. What is the implication of the formation of the 2nd product. Propose a possible mechanism for the reaction.
When optically pure 2,3-dimethyl-2pentanol was subjected to dehydration, a mixture of two alkenes was obtained. Hydrogenation of this alkene mixture gave 2,3-dimethylpentane. What were the two alkenes formed in the elimination reaction, and what were the relative amounts of each?
Are the molecules shown below chiral or achiral?
Are the molecules shown below chiral or achiral? Name the second compond.
Is the molecule shown below chiral or achiral?
Label each carbon atom as R or S
Label each carbon atom as R or S
How many asymmetric carbons are in the compound below.
How many asymmetric carbons are in the compound shown below?
You might also like
- Organic Chemistry Multiple Choice QuestionsDocument4 pagesOrganic Chemistry Multiple Choice QuestionsRonald Angelo LopezNo ratings yet
- Organic Chemistry: Zumdahl Chapter 22Document21 pagesOrganic Chemistry: Zumdahl Chapter 22Nurul AzizahNo ratings yet
- List of Cations and AnionsDocument1 pageList of Cations and AnionsAudrina Norbert77% (31)
- Balancing Activity - SkittlesDocument10 pagesBalancing Activity - SkittlesRhyz Mareschal DongonNo ratings yet
- Alkanes Cycloalkanes and AlkenesDocument3 pagesAlkanes Cycloalkanes and AlkenesDorota ZębikNo ratings yet
- Alkene Structure and PreparationDocument2 pagesAlkene Structure and PreparationCarlo Jay BasulNo ratings yet
- Chap 15-24Document49 pagesChap 15-24Linh NguyễnNo ratings yet
- Chapter 4 QuestionsDocument2 pagesChapter 4 Questionsdaniday1977100% (1)
- Tutorial 5Document4 pagesTutorial 5Eqieyn JerrNo ratings yet
- Chapter 7 QuestionsDocument4 pagesChapter 7 Questionsdaniday19770% (1)
- Tata Nama Alcohols and EthersDocument4 pagesTata Nama Alcohols and EthersAde RakhaNo ratings yet
- Document 1Document9 pagesDocument 1Nishi tomarNo ratings yet
- Tata Nama Alcohols and EthersDocument4 pagesTata Nama Alcohols and EthersNur ElidaNo ratings yet
- Exam Organic Chemistry I WhittenDocument19 pagesExam Organic Chemistry I WhittenDaniel Baylosis Asong60% (5)
- HW 1. StudentDocument3 pagesHW 1. Studentkitty2911No ratings yet
- Tata Nama Alcohols and EthersDocument4 pagesTata Nama Alcohols and Ethershana maghfyraNo ratings yet
- Organic ChemistryDocument7 pagesOrganic ChemistryPaul PGNo ratings yet
- Benzene and Derivatives Problem SetDocument2 pagesBenzene and Derivatives Problem SetCybrille Fleur Siobhan QúeensNo ratings yet
- IUPACDocument2 pagesIUPACAnubrata SarkarNo ratings yet
- Classfication and Nomeclature of Organic ComoundsDocument4 pagesClassfication and Nomeclature of Organic ComoundsEunbyeolNo ratings yet
- Chem Test 1Document5 pagesChem Test 1dishaali110No ratings yet
- Nomenclature Test 1Document7 pagesNomenclature Test 1Soham SagaonkarNo ratings yet
- IUPAC - Practice Sheet - IUPAC Nomenclature - ManzilDocument9 pagesIUPAC - Practice Sheet - IUPAC Nomenclature - ManzilShio100% (1)
- Alkanes Alkenes AlkynesDocument10 pagesAlkanes Alkenes AlkynesPanda Boy100% (2)
- CH 4 StudyDocument11 pagesCH 4 StudyLiz Hans0% (1)
- 61c3f345fc2e1700f41ec8be - ## - IUPAC Nomenclature: Homework PYQDocument29 pages61c3f345fc2e1700f41ec8be - ## - IUPAC Nomenclature: Homework PYQHhsjsNo ratings yet
- IUPAC Nomenclature - JEE TSC PDFDocument21 pagesIUPAC Nomenclature - JEE TSC PDFShadowNo ratings yet
- 3.1.1 Naming Practice QuestionsDocument33 pages3.1.1 Naming Practice QuestionsAyaan RaufNo ratings yet
- Date Planned: - / - / - Daily Tutorial Sheet-1 Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-1 Exact DurationDocument2 pagesDate Planned: - / - / - Daily Tutorial Sheet-1 Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-1 Exact DurationVIDYA SRI GANESHNo ratings yet
- Problem Set 2 Solution PDFDocument2 pagesProblem Set 2 Solution PDFLouisNo ratings yet
- Q2 2022 OrgchemDocument2 pagesQ2 2022 OrgchemHazeljoyce AlcantaraNo ratings yet
- Answer: CH H: IUPAC Name: C-Atom Classification: 1Document3 pagesAnswer: CH H: IUPAC Name: C-Atom Classification: 1Shaira Dawn PlancoNo ratings yet
- What Is The Total Number of Sigma Bonds Found in The Following CompoundDocument4 pagesWhat Is The Total Number of Sigma Bonds Found in The Following CompoundsubhasisknkNo ratings yet
- 2013 Homework KimorDocument23 pages2013 Homework KimorDiamondNadinaNo ratings yet
- Test Bank For Organic Chemistry 9Th Edition Carey Giuliano 0073402745 978007340274 Full Chapter PDFDocument17 pagesTest Bank For Organic Chemistry 9Th Edition Carey Giuliano 0073402745 978007340274 Full Chapter PDFclarence.kuhns728100% (11)
- Organic Chemistry 9th Edition Carey Giuliano Test BankDocument12 pagesOrganic Chemistry 9th Edition Carey Giuliano Test Bankmelissa100% (24)
- Bnbi104 Tutorial1Document1 pageBnbi104 Tutorial1Ashria Sonali PrakashNo ratings yet
- 13 DPP 2 New Batch A-N +ans For StudentsDocument32 pages13 DPP 2 New Batch A-N +ans For StudentskljNo ratings yet
- Quiz 01: Systematic NomenclatureDocument2 pagesQuiz 01: Systematic NomenclatureErica WuNo ratings yet
- DPP NomenclatureDocument7 pagesDPP Nomenclaturegamishtag18No ratings yet
- Organic Chemistry ReviewDocument2 pagesOrganic Chemistry ReviewLind MondanoNo ratings yet
- Alkanes, Alkenes, Alkynes and Their Alicyclic Couterparts: 1. What Is The IUPAC Name For CHDocument17 pagesAlkanes, Alkenes, Alkynes and Their Alicyclic Couterparts: 1. What Is The IUPAC Name For CHEllaŠtrbac100% (1)
- s.5 Chem TestDocument12 pagess.5 Chem TestlubaajamesNo ratings yet
- Anic Chemistry AK 2018-19Document22 pagesAnic Chemistry AK 2018-19XXXNo ratings yet
- 12 Pre Board 1 1222Document5 pages12 Pre Board 1 1222geyeyo9277No ratings yet
- Essential Organic Chemistry 2nd Edition Bruice Test BankDocument21 pagesEssential Organic Chemistry 2nd Edition Bruice Test Bankmarykirbyifsartwckp100% (15)
- Classification and Nomenclature: (Organic Chemistry)Document103 pagesClassification and Nomenclature: (Organic Chemistry)amar vnsNo ratings yet
- Classification and Nomenclature of Organic Compounds NJ 247Document29 pagesClassification and Nomenclature of Organic Compounds NJ 247gauravydv5306rgrNo ratings yet
- 768apni KakshaDocument31 pages768apni KakshaVimal PrasadNo ratings yet
- I UpacDocument19 pagesI UpacJay DodiyaNo ratings yet
- Ans ASC 0303 Tutorial 1 2015 Sem3 - AlkanesDocument15 pagesAns ASC 0303 Tutorial 1 2015 Sem3 - AlkanesJIEHASMARTNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetRehnuma BasherNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetValerie Duran-ArzagaNo ratings yet
- Organic Chemistry 9th Edition Carey Test BankDocument12 pagesOrganic Chemistry 9th Edition Carey Test BankCarolHutchinsonmrwjn100% (12)
- Nomenclature 1Document3 pagesNomenclature 1vmcuber1100% (1)
- 1 - Classification & Nomeclature of Organic CompoundsDocument8 pages1 - Classification & Nomeclature of Organic CompoundsarvindkrishnaNo ratings yet
- DPPS - 1 GocDocument4 pagesDPPS - 1 GocRajeev GangwarNo ratings yet
- Goc PDFDocument56 pagesGoc PDFManik SinghalNo ratings yet
- Analysis of Protein Post-Translational Modifications by Mass SpectrometryFrom EverandAnalysis of Protein Post-Translational Modifications by Mass SpectrometryNo ratings yet
- Novel Nanoscale Hybrid MaterialsFrom EverandNovel Nanoscale Hybrid MaterialsBhanu P. S. ChauhanNo ratings yet
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeFrom EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeNo ratings yet
- From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural ProductsFrom EverandFrom Biosynthesis to Total Synthesis: Strategies and Tactics for Natural ProductsNo ratings yet
- Groundwater Remediation: A Practical Guide for Environmental Engineers and ScientistsFrom EverandGroundwater Remediation: A Practical Guide for Environmental Engineers and ScientistsNo ratings yet
- Chapter+17 Multiple Choice QuestionsDocument4 pagesChapter+17 Multiple Choice QuestionsJosue UrciaNo ratings yet
- What Is The Difference Between Demineralized Water and Distilled WaterDocument2 pagesWhat Is The Difference Between Demineralized Water and Distilled WatervempadareddyNo ratings yet
- HydrocarbonDocument11 pagesHydrocarbonAida IshNo ratings yet
- Chemical Bonding II - VSEPR ModelDocument23 pagesChemical Bonding II - VSEPR ModelYokaris JTNo ratings yet
- Carbonyl Chemistry: Aim of CourseDocument65 pagesCarbonyl Chemistry: Aim of CourseEiann Jasper LongcayanaNo ratings yet
- Oxidation Reactions of Organic ChemistryDocument28 pagesOxidation Reactions of Organic ChemistryviejayNo ratings yet
- Chemical SegregationDocument4 pagesChemical SegregationLudy GiantoNo ratings yet
- Chemical Derivatization Dinesh 1 1Document12 pagesChemical Derivatization Dinesh 1 1Fatma MoustafaNo ratings yet
- 1 OctadeceneDocument3 pages1 OctadeceneJuan PadillaNo ratings yet
- Reference Tables For Physical Setting/CHEMISTRY: 2011 EditionDocument12 pagesReference Tables For Physical Setting/CHEMISTRY: 2011 EditionMaggieNo ratings yet
- Unit 1 Test ReviewDocument4 pagesUnit 1 Test Reviewandrew culkinNo ratings yet
- Multiple Choice Questions (MCQ) Topic Quiz 6.1 Aromatic Compounds, Carbonyls and AcidsDocument16 pagesMultiple Choice Questions (MCQ) Topic Quiz 6.1 Aromatic Compounds, Carbonyls and AcidsShaina Valiente GumallaoiNo ratings yet
- Ilovepdf Merged (2) RemovedDocument203 pagesIlovepdf Merged (2) RemovedalaaNo ratings yet
- Xi Chemistry QPDocument5 pagesXi Chemistry QPDamodar KasukurthiNo ratings yet
- Thermo ChemistryDocument18 pagesThermo ChemistryzaizazmkNo ratings yet
- Non AqueousDocument30 pagesNon AqueousDevananda R SNo ratings yet
- Expirement No 1Document4 pagesExpirement No 1Rajiv KharbandaNo ratings yet
- Latihan Fenol 1Document2 pagesLatihan Fenol 1MSMNo ratings yet
- Tutorial 1Document5 pagesTutorial 1nurqistina hanani HananNo ratings yet
- Problem SetsDocument7 pagesProblem SetsLouie G NavaltaNo ratings yet
- Aldehydes, Ketones and Carboxylic Acids. Aldehydes and KetonesDocument42 pagesAldehydes, Ketones and Carboxylic Acids. Aldehydes and KetonesVicky VigneshNo ratings yet
- 3E Chemical FormulaeDocument39 pages3E Chemical FormulaeAfida HamsaniNo ratings yet
- IodometriyDocument3 pagesIodometriySarvesh GaonkarNo ratings yet
- HydrocarbonsDocument5 pagesHydrocarbonsClaire Danes Tabamo DagalaNo ratings yet
- 3rd Seminar ReportDocument5 pages3rd Seminar Reportvaibhav palNo ratings yet
- Chemistry: NO CHODocument12 pagesChemistry: NO CHOPrasann KatiyarNo ratings yet
- Organic Chemistry - Worksheet 1Document10 pagesOrganic Chemistry - Worksheet 1Prakas PalanychamyNo ratings yet