Professional Documents
Culture Documents
Exercises For Lecture 6
Exercises For Lecture 6
Uploaded by
Tara EdwardsOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Exercises For Lecture 6
Exercises For Lecture 6
Uploaded by
Tara EdwardsCopyright:
Available Formats
Exercise for Lecture 6
A distillation column operating at 1 atm is to be designed for separating a binary mixture of A and B. The feed is 30 mass% A and the feed flow rate is 100 kg/hr of saturated liquid. A distillate composition of 90 mass% A and a bottoms composition of not more than 10 mass% A are desired. The values of HD and HB are 3390 and -770 kJ/kg, respectively. Using the Ponchon Savarit method, determine the number of ideal stages required to carry out the separation under these conditions. (Equilibrium and enthalpy data for the mixture at 1 atm pressure are given in the table). Note, HL refers to the enthalpy of the mixture at boiling point, HV refers to the enthalpy of the mixture at dew point.
T (oC) 110 105 101 98 96 95 92 88 84 82
xA 0
yA 0
HL (kJ/kg) 800 600 450 315 270 250 180 110 75 50
HV (kJ/kg) 2800 2700 2600 2510 2475 2450 2375 2300 2200 2125
0.09 0.33 0.18 0.59 0.26 0.73 0.3 0.4 0.55 0.7 0.8 0.77 0.85 0.9 0.94 0.96 0.32 0.79
81 80
0.9 1
0.98 1
30 25
2025 1900
Solution
H (kJ/kg)
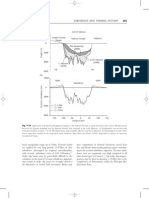
Plot the enthalpy line for liquid at boiling point (from the x and corresponding HL values) and vapour at dew point (from the y and corresponding HV values). Plot points M (xB,HB) and N (xD,HD) on this graph. Connect these points with a straight line and mark point F on the graph.
3500
3000
2500
2000
1500
1000
500
-500
M
-1000
0 0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
3500
Solution
3000 2500 2000 H (kJ/kg) 1500 1000 500 0 -500 -1000 0 1 0.9 0.8 0.7 yA 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 xA 0.6 0.7 0.8 0.9 1
Plot the equilibrium data (yA vs xA) and x = y line on a graph directly below the previous enthalpy graph.
M
0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
3500
Solution
3000 2500 2000 H (kJ/kg) 1500 1000 500 0 -500 -1000 0 1 0.9 0.8 0.7 yA 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 xA 0.6 0.7 0.8 0.9 1
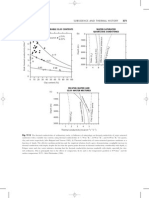
Beginning from the top of the column with xD, draw the operating line connecting point (xD,HDL) to point N. The point where this line crosses the dew point line indicates the vapour fraction and enthalpy of the vapour rising from the plate below, i.e. stage 1. The liquid on stage 1 is in equilibrium with the vapour on this stage. To find the liquid fraction, draw a vertical line down from y1 to meet the x = y line on the graph below. At the intersection draw a horizontal line to meet the equilibrium line. At the intersection draw a vertical line to meet the boiling point curve on the first graph. This gives x1, and a tie-line can join points x1 and y1.
M
0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
3500
Solution
3000 2500 2000 H (kJ/kg) 1500 1000 500 0 -500 -1000 0 1 0.9 0.8 0.7 yA 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 xA 0.6 0.7 0.8 0.9 1
Repeat the procedure for the next stage: draw the operating line connecting point (x1,H1L) to point N. The point where this line crosses the dew point line indicates the vapour fraction and enthalpy of the vapour rising from the plate below, i.e. stage 2. The liquid on stage 2 is in equilibrium with the vapour on this stage. To find the liquid fraction, draw a vertical line down from y2 to meet the x = y line on the graph below. At the intersection draw a horizontal line to meet the equilibrium line. At the intersection draw a vertical line to meet the boiling point curve on the first graph. This gives x2, and a tie-line can join points x2 and y2.
M
0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
3500
Solution
3000 2500 2000 H (kJ/kg) 1500 1000 500 0 -500 -1000 0 1 0.9 0.8 0.7 yA 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 xA 0.6 0.7 0.8 0.9 1
Repeat the procedure for the next stage: draw the operating line connecting point (x2,H2L) to point N. The point where this line crosses the dew point line indicates the vapour fraction and enthalpy of the vapour rising from the plate below, i.e. stage 3. The liquid on stage 3 is in equilibrium with the vapour on this stage. To find the liquid fraction, draw a vertical line down from y3 to meet the x = y line on the graph below. At the intersection draw a horizontal line to meet the equilibrium line. At the intersection draw a vertical line to meet the boiling point curve on the first graph. This gives x3, and a tie-line can join points x3 and y3.
M
0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
3500
Solution
3000 2500 2000 H (kJ/kg) 1500 1000 500 0 -500 -1000 0 1 0.9 0.8 0.7 yA 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 xA 0.6 0.7 0.8 0.9 1
Since the liquid fraction on stage 3 is smaller than that in the feed, this corresponds to the stripping section. Draw the operating line connecting point (x3,H3L) to point M. Extend this line to the dew point line. The point where this line crosses the dew point line indicates the vapour fraction and enthalpy of the vapour rising from the plate below, i.e. stage 4. To find the liquid fraction, draw a vertical line down from y4 to meet the x = y line on the graph below. At the intersection draw a horizontal line to meet the equilibrium line. At the intersection draw a vertical line to meet the boiling point curve on the first graph. This gives x4, and a tie-line can join points x4 and y4.
M
0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
3500
Solution
3000 2500 2000 H (kJ/kg) 1500 1000 500 0 -500 -1000 0 1 0.9 0.8 0.7 yA 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 xA 0.6 0.7 0.8 0.9 1
Repeat the procedure for the next stage: draw the operating line connecting point (x4,H4L) to point M. The point where this line crosses the dew point line indicates the vapour fraction and enthalpy of the vapour rising from the plate below, i.e. stage 5. The liquid on stage 5 is in equilibrium with the vapour on this stage. To find the liquid fraction, draw a vertical line down from y5 to meet the x = y line on the graph below. At the intersection draw a horizontal line to meet the equilibrium line. At the intersection draw a vertical line to meet the boiling point curve on the first graph. This gives x5, and a tie-line can join points x5 and y5.
M
0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
3500
Solution
3000 2500 2000 H (kJ/kg) 1500 1000 500 0 -500 -1000 0 1 0.9 0.8 0.7 yA 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 xA 0.6 0.7 0.8 0.9 1
Since the liquid fraction on stage 5 is smaller than that required in the bottom product, this stage (stage 5) is the final equilibrium stage required.
M
0.1 0.2 0.3 0.4 0.5 xA, yA 0.6 0.7 0.8 0.9 1
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Executive Summary For GulaimDocument2 pagesExecutive Summary For GulaimTara EdwardsNo ratings yet
- Permeabilities Key MDDocument8 pagesPermeabilities Key MDTara EdwardsNo ratings yet
- The Oil Depletion ProtocolDocument53 pagesThe Oil Depletion ProtocolTara EdwardsNo ratings yet
- Example 1: Underwood & Fenske equations: Component α x x xDocument8 pagesExample 1: Underwood & Fenske equations: Component α x x xTara EdwardsNo ratings yet
- WattenbergDocument38 pagesWattenbergTara EdwardsNo ratings yet
- Toluene DisproportionDocument4 pagesToluene DisproportionTara EdwardsNo ratings yet
- Depth Section: Nondeposition Basin FloorDocument0 pagesDepth Section: Nondeposition Basin FloorTara EdwardsNo ratings yet
- Exercises For Lecture x2Document8 pagesExercises For Lecture x2Tara EdwardsNo ratings yet
- Separation Processes - Tutorial 3: DR Colin HareDocument7 pagesSeparation Processes - Tutorial 3: DR Colin HareTara EdwardsNo ratings yet
- Sedimentology Lecture Notes (Week 5 Test)Document4 pagesSedimentology Lecture Notes (Week 5 Test)Tara EdwardsNo ratings yet
- 8.4.1 Carbonate StratigraphyDocument0 pages8.4.1 Carbonate StratigraphyTara EdwardsNo ratings yet
- Esogenesis and Telogenesis of Sandstones: Cements Such As Chlorite, Which May Inhibit QuartzDocument0 pagesEsogenesis and Telogenesis of Sandstones: Cements Such As Chlorite, Which May Inhibit QuartzTara EdwardsNo ratings yet
- Application To Petroleum Play AssessmentDocument0 pagesApplication To Petroleum Play AssessmentTara EdwardsNo ratings yet
- Fig. 9.10 Application of Flexural Backstripping Technique To The Valencia Trough, A Young Rift Basin in The Western MediterraneanDocument0 pagesFig. 9.10 Application of Flexural Backstripping Technique To The Valencia Trough, A Young Rift Basin in The Western MediterraneanTara EdwardsNo ratings yet
- Water-Saturated Quartzose Sandstones: Subsidence and Thermal HistoryDocument0 pagesWater-Saturated Quartzose Sandstones: Subsidence and Thermal HistoryTara EdwardsNo ratings yet
- Mesogenetic (Beyond The Influence of Surficial Processes) Karst Systems That Enhance Vertical Conductivity of FluidsDocument0 pagesMesogenetic (Beyond The Influence of Surficial Processes) Karst Systems That Enhance Vertical Conductivity of FluidsTara EdwardsNo ratings yet
- PEME2210 Engineering Science 2 Heat Transfer: Tutorial 1 (Solution)Document2 pagesPEME2210 Engineering Science 2 Heat Transfer: Tutorial 1 (Solution)Tara EdwardsNo ratings yet
- Gravimetric Analysis01Document13 pagesGravimetric Analysis01M AzeemNo ratings yet
- Modern Theories of Acids & Bases: The Arrhenius and Bronsted-Lowry TheoriesDocument48 pagesModern Theories of Acids & Bases: The Arrhenius and Bronsted-Lowry TheoriesAgung PratamaNo ratings yet
- Manual For Calculating Distribution CoefficientDocument3 pagesManual For Calculating Distribution CoefficientMohit JagtapNo ratings yet
- On Acids, Bases & Salts Submitted by Bhavneet SinghDocument27 pagesOn Acids, Bases & Salts Submitted by Bhavneet SinghBhavneet SinghNo ratings yet
- Polyprotic Acid-Base EquilibriaDocument2 pagesPolyprotic Acid-Base EquilibriaNicos RiveraNo ratings yet
- Apsc 183 (2019W-T2) T4Document10 pagesApsc 183 (2019W-T2) T4Sara 123No ratings yet
- Linearity Study On Detection and Quantification LiDocument6 pagesLinearity Study On Detection and Quantification LiLaura GuarguatiNo ratings yet
- Analytical Chemistry by D. Kealey and P. J. HainesDocument353 pagesAnalytical Chemistry by D. Kealey and P. J. HainessussanNo ratings yet
- Definitions - Topic 12 Acid-Base Equilibria - Edexcel Chemistry A-LevelDocument2 pagesDefinitions - Topic 12 Acid-Base Equilibria - Edexcel Chemistry A-LevelsalmaNo ratings yet
- 3.0 Results Experiment 2Document3 pages3.0 Results Experiment 2Salihah AbdullahNo ratings yet
- Chapter IIDocument211 pagesChapter IIjonida88No ratings yet
- 422 Sol 26Document5 pages422 Sol 26Merna El SayeghNo ratings yet
- CHM110 Lab Final Exam Type ADocument3 pagesCHM110 Lab Final Exam Type Areachifeanyi100% (1)
- Quiz 4.2Document3 pagesQuiz 4.2Annabelle Cebrero Paulo AlbaoNo ratings yet
- Notes of CH 2 Acids, Bases and Salts - Class 10th Science Study RankersDocument5 pagesNotes of CH 2 Acids, Bases and Salts - Class 10th Science Study Rankerssamy100% (1)
- PhET Lab - Acid Base SolutionsDocument3 pagesPhET Lab - Acid Base SolutionsuwantnovaporNo ratings yet
- Comparative Study of Chelating Ion Exchange Resins For The Recovery of Nickel and Cobalt From Laterite Leach Tailings PDFDocument5 pagesComparative Study of Chelating Ion Exchange Resins For The Recovery of Nickel and Cobalt From Laterite Leach Tailings PDFRodrigoNo ratings yet
- Vce Chemistry Unit 3 Sac 2 Equilibrium Experimental Report: InstructionsDocument5 pagesVce Chemistry Unit 3 Sac 2 Equilibrium Experimental Report: InstructionsJefferyNo ratings yet
- Mulia Sejahtera ScientificDocument10 pagesMulia Sejahtera Scientificfauzi.muhammad143No ratings yet
- Iodimetric Titration: Aim: PrincipleDocument2 pagesIodimetric Titration: Aim: PrincipleHarsh ThakurNo ratings yet
- I. About Graphs: What Is A Graph?Document29 pagesI. About Graphs: What Is A Graph?Mae DinDinNo ratings yet
- 10X RIPA Buffer Ab156034 10X RIPA Buffer Ab156034: Product Datasheet Product DatasheetDocument2 pages10X RIPA Buffer Ab156034 10X RIPA Buffer Ab156034: Product Datasheet Product DatasheetRicardo PonceNo ratings yet
- Karl FischerDocument9 pagesKarl FischerArunima GhoseNo ratings yet
- MODULE 2.4. ACIDS AND BASES - Lecture, No Animations - PDFDocument23 pagesMODULE 2.4. ACIDS AND BASES - Lecture, No Animations - PDFhey yutNo ratings yet
- Unit 1 Crystal Structure: Palak Desai Mechanical Engineering DepartmentDocument44 pagesUnit 1 Crystal Structure: Palak Desai Mechanical Engineering DepartmentPalak NaikNo ratings yet
- AP CHEM Lab Acid Base Titration PDFDocument4 pagesAP CHEM Lab Acid Base Titration PDFMelnykNo ratings yet
- Accumulated Deposition in A Steam Generator Tube: Standard Test Methods ForDocument7 pagesAccumulated Deposition in A Steam Generator Tube: Standard Test Methods ForMaxNo ratings yet
- Solid State 1 MR DavidDocument188 pagesSolid State 1 MR Davidfrank samndomiNo ratings yet
- Quantitative Elemental Analysis of Sodium (Na) by NMR SpectrosDocument2 pagesQuantitative Elemental Analysis of Sodium (Na) by NMR SpectrosRachid MakhloufiNo ratings yet
- Acids Bases and Salts WorksheetDocument4 pagesAcids Bases and Salts WorksheetMelva GuerraNo ratings yet