Professional Documents
Culture Documents
Lugol's Solution (Essentially Iodine)
Lugol's Solution (Essentially Iodine)
Uploaded by
serena rodriguezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lugol's Solution (Essentially Iodine)
Lugol's Solution (Essentially Iodine)
Uploaded by
serena rodriguezCopyright:
Available Formats
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
Lugols solution (essentially iodine)
*Binds to helical structure of starch (iodines
are the faded purple spheres above), turning
the substance or solution a blue/black, but
only binds because structure is helical.
Therefore, will it bind
glucose?
explain
No,to
it isnt
helical and
therefore
the solution will not turn blue-
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
Lugols solution (essentially iodine)
What is lugols iodine solution?
Conclusion: Lugols solution is a starch
indicator
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
1. Making the model cell
Dialysis tubing (see below)
A synthetic membrane that has microscopic pores (holes) that only let
molecules of a certain size or smaller to pass. The one we are using only allows
monomers or smaller molecules to pass.
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
1. Making the model cell
Dialysis tubing
A synthetic membrane that has microscopic pores (holes) that only let
molecules of a certain size or smaller to pass. The one we are using only allows
monomers or smaller molecules to pass.
If you soak it in water, you
will be able to open up the
tubing to put solution
inside. Before you can do
this, however, you must
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
1. Making the model cell
Dialysis tubing
A synthetic membrane that has microscopic pores (holes) that only let
molecules of a certain size or smaller to pass. The one we are using only allows
monomers or smaller molecules to pass.
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
1. Making the model cell
Dialysis tubing
A synthetic membrane that has microscopic pores (holes) that only let
molecules of a certain size or smaller to pass. The one we are using only allows
monomers or smaller molecules to pass.
Glucose, starch, water
Then put
glucose and
starch
solution in
bag
Artificial Cell
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
2. Place the model cell into a solution containing lugols
(iodine).
Predict what should happen:
1. The glucose should diffuse out down its
I-
I-
Glucose, starch, water
I-
Artificial Cell
concentration gradient and the iodine should
diffuse in down its concentration gradient since
both can pass through the membrane pores. The
starch should stay in too large to pass through
pores. Water should diffuse in and out direction
will depend on how much solute you dissolved in
2. How
will you observe that the iodine
the
water.
I-
went
Thein?
iodine should bind to the helical starch structure
causing the solution inside the dialysis tubing to turn
blue-black.
3. How will you observe that the starch did
not diffuse
The outside
out?
should remain amber (the color of
I-
lugols) since there is no starch present to react with
it.
4. How do we observe that glucose diffused
out?
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
Use Benedicts solution
- Benedicts is a molecule that reflects blue light that will react with
monosaccharides, lactose and maltose and convert into a molecule
that reflects yellow/orange light. The reaction is endergonic. How we
canYou
we must
get it heat
to happen?
the Benedicts in the presence of the sugars. Input
ENERGY!
1. Add a few
drops of
benedicts to
the solution
2. MUST HEAT!!
3. Turns orange
if
monosaccharid
es, lactose or
maltose is
present
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
2. Place the model cell into a solution containing lugols
(iodine).
How do we observe that glucose diffused out?
I-
II-
Glucose, starch, water
I-
Artificial Cell
Take a sample of the outer solution into a
clean test tube, add a few drops of benedicts,
and heat it up. If it turns orange then it
diffused out.
I-
I-
Time to do it!
I-
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
II-
Glucose, starch, water
Q. You have a solution containing 1mM catalase. The pH of the solution is around
6.8 and the salt (NaCl) concentration is around 400 mM (milli Molar). However, you
have previously determined that this protein functions best at a pH of 5.7 and a salt
concentration of 250 mM. Explain how you can get your protein into the desired
conditions without changing the concentration of the enzyme much.
Take a sample of the outer solution into a
clean test tube, add a few drops of benedicts,
IIand heat it up. If it turns orange then it
diffused out.
I
Artificial Cell
I-
Time to do it!
I-
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
Q. You have a solution containing 1mM catalase. The pH of the solution is around
6.8 and the salt (NaCl) concentration is around 400 mM (milli Molar). However, you
have previously determined that this protein functions best at a pH of 5.7 and a salt
concentration of 250 mM. Explain how you can get your protein into the desired
conditions without changing the concentration of the enzyme much.
Diffusion Through a Membrane State Lab
Part 1 Build a model cell and demonstrate diffusion across
a selectively permeable membrane
Q. You have a solution containing 1mM catalase. The pH of the solution is around
6.8 and the salt (NaCl) concentration is around 400 mM (milliMolar). However, you
have previously determined that this protein functions best at a pH of 5.7 and a salt
concentration of 250 mM. Explain how you can get your protein into the desired
conditions without changing the concentration of the enzyme much.
Catalase solution
with pH 6.8 and
400mM NaCl
Aqueous solution with
pH 5.7 and 250 mM
NaCl
A. Use a dialysis tube. Put your protein
solution into the dialysis membrane and tie it
off. Place the membrane into the desired
solution. Salt will diffuse out and H+ will
diffuse in. Protein will stay inside as it is too
large to fit through pores
This is called Buffer
Diffusion Through a Membrane State Lab
Part 2 Demonstrate diffusion of water across a real
membrane (osmosis)
You want to observe the effect of
salt on osmosis across the plasma
membrane of red onion cells under
the light microscope. How will you
do this?
You look at the cells in water first.
control group
This is your______________.
All of the cells should beturgid
_____________.
What will you do next?
Red onion cells in water (turgid) Add salt to the solution that the
cells are in. What would you
expect to happen and why?
Diffusion Through a Membrane State Lab
Part 2 Demonstrate diffusion of water across a real
membrane (osmosis)
Add salt
(NaCl)
Red onion cells in water (turgid)
plasmolysis
Osmosis (diffusion of water) should occur from inside the cell [high] to outside [low] since the
salt CANNOT cross the membrane and will cause the water concentration outside the cell to
now lower than insideyou should observe plasmolysis. How do we make these cells turgid
Diffusion Through a Membrane State Lab
Part 2 Demonstrate diffusion of water across a real
membrane (osmosis)
Remove
salt (NaCl)
Red onion cells in water (turgid)
plasmolysis
Put them back into tap water (get rid of the salt).
Diffusion Through a Membrane State Lab
Part 2 Demonstrate diffusion of water across a real
membrane (osmosis)
Remove
salt (NaCl)
Red onion cells in water (turgid)
plasmolysis
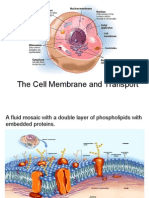
Remember: This is a real cell membrane (phospholipid bilayer with integral membrane
protein), which will only allow small hydrophobic molecules to pass (you should know
why). Sodium chloride (Na+, Cl-) is charged and hence hydrophilic.
You might also like
- Answer Key Lab Diffusion and OsmosisDocument8 pagesAnswer Key Lab Diffusion and OsmosissabrinaNo ratings yet
- Animal Cell Parts and Functions - Summary TableDocument16 pagesAnimal Cell Parts and Functions - Summary TableAnnie Lyn Villamor100% (1)
- Diffusion and Osmosis LabDocument13 pagesDiffusion and Osmosis Labapi-233777623No ratings yet
- Oncologic Nursing ReviewerDocument4 pagesOncologic Nursing ReviewershaynehonoreeNo ratings yet
- Ascension Workbook IIDocument468 pagesAscension Workbook IIElizabeth Rose94% (17)
- Dialysis ExperimentDocument2 pagesDialysis ExperimentAbhishek GadhwalNo ratings yet
- Cell Membranes & Transport 1Document77 pagesCell Membranes & Transport 1Alen SheenNo ratings yet
- Lab Report 1Document5 pagesLab Report 1buiiianna1995No ratings yet
- Laboratory Experiment 1Document6 pagesLaboratory Experiment 1Rutchelle Desiree SenadonNo ratings yet
- Activity Lab 1Document10 pagesActivity Lab 1Chamae IsagaNo ratings yet
- Exercise No. 1 Movement of Substance Through Cell Membrane Experiment Demonstrating DiffusionDocument8 pagesExercise No. 1 Movement of Substance Through Cell Membrane Experiment Demonstrating DiffusionBernadette VillacampaNo ratings yet
- Biology 110 Principles of Biology 17 3. Diffusion and OsmosisDocument10 pagesBiology 110 Principles of Biology 17 3. Diffusion and OsmosislauraningsihNo ratings yet
- Biochem 1st LabDocument12 pagesBiochem 1st LabDarl MalazarteNo ratings yet
- Osmosis LabDocument2 pagesOsmosis LabCorsea McLaughlinNo ratings yet
- An Introduction To Cell Structure & Function: CellsDocument14 pagesAn Introduction To Cell Structure & Function: Cellsyasser alozaibNo ratings yet
- IGCSE Edexcel (9-1) H.Biology Notes: 1. Cells and TissuesDocument7 pagesIGCSE Edexcel (9-1) H.Biology Notes: 1. Cells and TissuesnaduniNo ratings yet
- Physiology Lab 1 2Document11 pagesPhysiology Lab 1 2tmqt2fbnzgNo ratings yet
- Cell Membrane and Transport Lab Teacher Activity GuideDocument8 pagesCell Membrane and Transport Lab Teacher Activity GuideSayy MaeNo ratings yet
- Lab 4 PP 25-31 WMtWeec PDFDocument7 pagesLab 4 PP 25-31 WMtWeec PDFTAHA GABRNo ratings yet
- Labbio Sat TrangQuynhKhoiVy Labreport 1 4Document10 pagesLabbio Sat TrangQuynhKhoiVy Labreport 1 4Quynh Dang PhuongNo ratings yet
- Module 3 - Melendres - Bianca OutputDocument7 pagesModule 3 - Melendres - Bianca OutputBianca MelendresNo ratings yet
- Lab 5 Cell Membrane Structure and Function FinalDocument12 pagesLab 5 Cell Membrane Structure and Function Finalannekemp100% (2)
- AbcDocument3 pagesAbcAnnedi ZonNo ratings yet
- CellDocument5 pagesCellMyrene CeblanoNo ratings yet
- Cell Defense WorksheetDocument3 pagesCell Defense WorksheetAdriana UrquiaNo ratings yet
- 9 Fundmentals of LifeDocument8 pages9 Fundmentals of LifehayzkidsNo ratings yet
- Biology Laboratory: School of BiotechnologyDocument8 pagesBiology Laboratory: School of BiotechnologyNgọc Phương Anh NguyễnNo ratings yet
- Progress Indicators: Learning Objective: To Understand The Structure of Animal and Plant CellsDocument49 pagesProgress Indicators: Learning Objective: To Understand The Structure of Animal and Plant CellsnidhiNo ratings yet
- AP Bio Lab1Document10 pagesAP Bio Lab1Eva DesireeNo ratings yet
- AQA Biology As Condensed Notes - Chapter 3, Cell Structure and MembranesDocument19 pagesAQA Biology As Condensed Notes - Chapter 3, Cell Structure and MembranesSairah RazakNo ratings yet
- Lab 5 Osmosis and TonicityDocument6 pagesLab 5 Osmosis and TonicityKathleen RussellNo ratings yet
- StatelabdiffusionDocument30 pagesStatelabdiffusionfieldbkNo ratings yet
- 2 Structure and Functions in Living OrganismsDocument36 pages2 Structure and Functions in Living OrganismsSam ShohetNo ratings yet
- Diffusion Through A Membrane Lab Review SheetDocument2 pagesDiffusion Through A Membrane Lab Review SheetTheresa SullyNo ratings yet
- Osmosis Simulation Through The Cell MembraneDocument12 pagesOsmosis Simulation Through The Cell Membraneapi-345382516No ratings yet
- Diffusion and Osmosis PPT ACDocument26 pagesDiffusion and Osmosis PPT ACissacabhishekdaraNo ratings yet
- Unit 2 AP Lab #3Document10 pagesUnit 2 AP Lab #3laraNo ratings yet
- CH 05 Cell Membrane and TransportDocument20 pagesCH 05 Cell Membrane and Transportapi-254384607No ratings yet
- General Biology Lab Bio 105 / 112: Lab # 6 Physical Properties of CellsDocument30 pagesGeneral Biology Lab Bio 105 / 112: Lab # 6 Physical Properties of CellsA-Naeem To'mah Al-sawaieNo ratings yet
- AP Bio CH 7 Lab SummaryDocument9 pagesAP Bio CH 7 Lab SummaryBrian HerrmannNo ratings yet
- Review Questions Plasma Membrane 1. What Is The Function of The Plasma Membrane?Document11 pagesReview Questions Plasma Membrane 1. What Is The Function of The Plasma Membrane?Jennie Rose KomalaNo ratings yet
- BCHO3L-The-Biomolecules-in-Some-Cell-Organelles 2Document8 pagesBCHO3L-The-Biomolecules-in-Some-Cell-Organelles 2obicopenelopeNo ratings yet
- Chapter - 04 - The Cell in ActionDocument22 pagesChapter - 04 - The Cell in ActionBily roriguesNo ratings yet
- Cell Membrane and Transport Lab Teacher Activity GuideDocument9 pagesCell Membrane and Transport Lab Teacher Activity GuideDorothy Joyce DXNo ratings yet
- Lab 4 Dialysis ManualDocument8 pagesLab 4 Dialysis Manualassem.assilbekovaNo ratings yet
- Lab Exercise 3: Membrane Transport Mechanisms and Osmosis The Cell CycleDocument13 pagesLab Exercise 3: Membrane Transport Mechanisms and Osmosis The Cell CyclerezamaulanaNo ratings yet
- Exercise 1Document14 pagesExercise 1Michelle ViduyaNo ratings yet
- BIOLOGYLOVEDocument43 pagesBIOLOGYLOVEOrkid Fazz93% (14)
- Science Short Notes Grade 10Document32 pagesScience Short Notes Grade 10Mallindu PereraNo ratings yet
- Ellc Rmnbemae Llce Bmmneera Lecl Mebmaren Lcel Nbmemaer: Cell MembraneDocument9 pagesEllc Rmnbemae Llce Bmmneera Lecl Mebmaren Lcel Nbmemaer: Cell MembraneAlvin PaboresNo ratings yet
- BIOL1408 Introductory Biology Name Lab Unit 6/7: Diffusion & Osmosis Date Dr. Flo OxleyDocument10 pagesBIOL1408 Introductory Biology Name Lab Unit 6/7: Diffusion & Osmosis Date Dr. Flo OxleyJavier E. Dubon0% (1)
- Movement of Subtances Across Plasma MembraneDocument414 pagesMovement of Subtances Across Plasma MembranezazaNo ratings yet
- Osmosis Diffusion Lab-1Document7 pagesOsmosis Diffusion Lab-1api-1658690000% (1)
- Experiment 1A The CellDocument3 pagesExperiment 1A The CellLeah SunioNo ratings yet
- Cell Physiology: Exercise IDocument10 pagesCell Physiology: Exercise Iangela mamauagNo ratings yet
- Cells and Membranes Notes For WebsiteDocument28 pagesCells and Membranes Notes For Websiteapi-292966101No ratings yet
- Report 1: Practical 1: Microscopy, Cell Observation & OsmosisDocument8 pagesReport 1: Practical 1: Microscopy, Cell Observation & OsmosisDương Hà Trúc TâmNo ratings yet
- Cell Diffusion & Permeability - Teacher VersionDocument9 pagesCell Diffusion & Permeability - Teacher VersionTeachLABScINo ratings yet
- LAB 9 - ChromatographyDocument20 pagesLAB 9 - ChromatographyAnothando GobaNo ratings yet
- Chapter 5Document3 pagesChapter 5eggswim50% (2)
- Stootsd UnitplanreflectionDocument3 pagesStootsd Unitplanreflectionapi-317044149No ratings yet
- 9 BioDocument3 pages9 BioAnubhav jhaNo ratings yet
- Chapter 7 Test Bank: Multiple ChoiceDocument22 pagesChapter 7 Test Bank: Multiple ChoicePayal100% (1)
- Edible Cell Biology Project RubricDocument1 pageEdible Cell Biology Project Rubricalathena alathenaNo ratings yet
- Ib Biology SLDocument68 pagesIb Biology SLShelley Lima100% (1)
- 2014 2 2 3 EnwaDocument10 pages2014 2 2 3 EnwaAdela Cynthia AltairaNo ratings yet
- Mocking HedtfeDocument95 pagesMocking HedtfeOlniNo ratings yet
- IceAwake 201912Document30 pagesIceAwake 201912NG Sze WingNo ratings yet
- NEET 2016 Question Paper Phase 2 Code AA PP WW1Document19 pagesNEET 2016 Question Paper Phase 2 Code AA PP WW1Dev ranjanNo ratings yet
- Biol1130 2016 Sem-1 CrawleyDocument4 pagesBiol1130 2016 Sem-1 CrawleyDoonkie100% (1)
- Topic 6 - Cell Cycle and MitosisDocument55 pagesTopic 6 - Cell Cycle and MitosisJJNo ratings yet
- Cell City Restaurant: Danielle RiddleDocument2 pagesCell City Restaurant: Danielle RiddleLMNo ratings yet
- MR Supple All 2014Document429 pagesMR Supple All 2014Regita Ayu LestariNo ratings yet
- Powerpoint Pembelajaran Bahasa Inggris SMA Kelas XII 2018Document89 pagesPowerpoint Pembelajaran Bahasa Inggris SMA Kelas XII 2018Johana Wulandari75% (8)
- Concepts and Theories of Aging: Princy Francis M I Yr MSC (N) JmconDocument48 pagesConcepts and Theories of Aging: Princy Francis M I Yr MSC (N) Jmconcj bariasNo ratings yet
- BSC ParamedicalDocument1 pageBSC ParamedicalUmair KhanNo ratings yet
- Exam Questions 2nd October With MsDocument12 pagesExam Questions 2nd October With MsThe Man Behind The SlaughterNo ratings yet
- Environmental Science TermsDocument40 pagesEnvironmental Science TermsNasraRealinoNo ratings yet
- Ncert Solutions Feb 2021 Class 10 English Supplementary Footprints Without Feet Chapter 6Document3 pagesNcert Solutions Feb 2021 Class 10 English Supplementary Footprints Without Feet Chapter 6NAWIN AMIRTHARAJNo ratings yet
- ZoologyDocument97 pagesZoologyEnsNo ratings yet
- Prescott's: MicrobiologyDocument9 pagesPrescott's: Microbiologyethan jasonNo ratings yet
- NCSE Syllabus PDFDocument125 pagesNCSE Syllabus PDFDarryan Dhanpat100% (1)
- Cheek and Onion Cell LabDocument6 pagesCheek and Onion Cell Labapi-259776843No ratings yet
- Biological ClassificationDocument327 pagesBiological ClassificationpotqatocakesiiNo ratings yet
- TaxonomyDocument56 pagesTaxonomyKrezia Mae SolomonNo ratings yet
- Chapter 1 - Introduction: Biology TodayDocument16 pagesChapter 1 - Introduction: Biology TodayJordan JunotNo ratings yet
- Aja Exam Number 4Document2 pagesAja Exam Number 4Myla Angelica AndresNo ratings yet