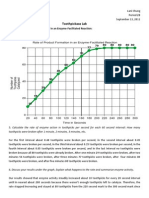
Professional Documents
Culture Documents
MBD Toolkit A
MBD Toolkit A
Uploaded by
OCRChemistrySaltersOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MBD Toolkit A
MBD Toolkit A
Uploaded by
OCRChemistrySaltersCopyright:
Available Formats
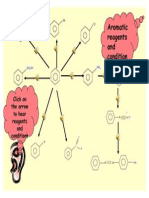
esterification
oxidation oxidation
elimination
R1OH / c.H2SO4 catalyst O
R H O O
Cr2O72- / H+(aq) / reflux Cr2O72- / H+(aq)
C C Al2O3(s) / 300C R C R C reflux R C
R CH2 OH
H reflux OH
H H or c.H2SO4 / reflux H (aq) / H2O
+
O R1
alcohol NaBH4
alkene followed by H2O aldehyde carboxylic acid ester
reduction hydrolysis
substitution substitution
acylation
HBr(aq) NaOH(aq) NaBr(s) / c.H2SO4 substitution
room temp reflux R1OH
reflux
room temp
addition SCl2O
reflux
NaCN in aqueous O O
addition
ethanol solution c.NH3(aq)
R CH2 Br R CH2 CN R C R C
halogenoalkane reflux nitrile Cl room temp NH2
H2(g)/Ni acyl chloride acylation primary amide
substitution
150C
5 atm substitution
hydrolysis
acylation
Br2(l) substitution
sunlight R1NH2
c. NH3(aq) H+(aq)/H2O
room temp
heat in a sealed tube reflux
O
R CH3 R CH2 NH 2 R CH2 COOH R C
alkane amine carboxylic acid
(reacts as above) NH R1
secondary amine
Notes: 1. The formation of a nitrile from a halogenoalkane is a carbon-carbon bond forming reaction.
The carboxylic acid formed from the nitrile has an extra carbon atom in the side-chain.
All the other reactions are simple functional group interconversions.
2. The halogenoalkane shown will only be a minor product of the reaction from the alkene.
The main product will be the isomer with the Br atom attached to the second carbon atom.
3. You may wish to add other reactions to this toolkit. For example the formation of a secondary
alcohol from an alkene RCH=CHR1 and its subsequent oxidation to the ketone.
You might also like
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- Ib Chem IaDocument9 pagesIb Chem IaFrank Lala0% (1)
- A2-Organic Reactions Spider Diagram HANDOUT (Colour)Document1 pageA2-Organic Reactions Spider Diagram HANDOUT (Colour)udaymohur100% (1)
- Synthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsDocument6 pagesSynthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsJunior GonzalesNo ratings yet
- Biology - Toothpickase LabDocument3 pagesBiology - Toothpickase LablanichungNo ratings yet
- 14.1 Multiple-Choice Questions: Chapter 14 Chemical KineticsDocument43 pages14.1 Multiple-Choice Questions: Chapter 14 Chemical KineticsanonNo ratings yet
- Chemistry Handbook ExportDocument17 pagesChemistry Handbook Exportgauranggunjkar2006No ratings yet
- Reaction of Alcohols CompleteDocument1 pageReaction of Alcohols CompleteJoko SusiloNo ratings yet
- Chemistry - Overview of Aliphatic Organic ChemistryDocument1 pageChemistry - Overview of Aliphatic Organic Chemistryhelixate100% (5)
- Aliphatic Organic Chemistry Mindmap PDFDocument1 pageAliphatic Organic Chemistry Mindmap PDFIsuru ThenabaduNo ratings yet
- Organic Chemistry Reaction Summary SheetDocument30 pagesOrganic Chemistry Reaction Summary SheetKylo RenNo ratings yet
- Organic Chemistry PosterDocument1 pageOrganic Chemistry Poster텅텅No ratings yet
- X UV Light or Heat: Reactions in Topic XIDocument3 pagesX UV Light or Heat: Reactions in Topic XImichelsonyip100% (1)
- Reactions of Alkene: CH CH Markovnikov AdditionDocument8 pagesReactions of Alkene: CH CH Markovnikov AdditionRaye VolvoNo ratings yet
- OXIDATIONS FinalDocument9 pagesOXIDATIONS Finalgamer boomerNo ratings yet
- 2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Document17 pages2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Anarkin FitriNo ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidSsNo ratings yet
- Reaction of Carboxylic Acid CompleteDocument1 pageReaction of Carboxylic Acid CompleteJoko SusiloNo ratings yet
- Biodegradation: Dr. Stephen Johnson S.j.johnson@gre - Ac.ukDocument32 pagesBiodegradation: Dr. Stephen Johnson S.j.johnson@gre - Ac.ukaziskfNo ratings yet
- Memory Map AromaticsDocument1 pageMemory Map AromaticsOCRChemistrySaltersNo ratings yet
- Screenshot 2024-04-16 at 22.57.21Document6 pagesScreenshot 2024-04-16 at 22.57.21fpjbrqfyktNo ratings yet
- Alcohols CIE 9701 As Level Reaction Scheme 1Document1 pageAlcohols CIE 9701 As Level Reaction Scheme 1Daniel MulipolaNo ratings yet
- Road Map Organic PDFDocument5 pagesRoad Map Organic PDFS SquareNo ratings yet
- SynrxnsDocument48 pagesSynrxnsRonak MantriNo ratings yet
- EtherDocument1 pageEtherBao TranNo ratings yet
- Reaction of AldehydesDocument1 pageReaction of AldehydesJoko SusiloNo ratings yet
- Allen Organic QUICK RevisionDocument2 pagesAllen Organic QUICK RevisionChetna Ahlawat100% (2)
- Lecture 13 OrgDocument7 pagesLecture 13 OrgBikram GhoshNo ratings yet
- Reaction of Ketone CompleteDocument1 pageReaction of Ketone CompleteJoko SusiloNo ratings yet
- Organic Synthesis Reaction Pathways - A Level ChemistryDocument15 pagesOrganic Synthesis Reaction Pathways - A Level ChemistryremesanmeenakshiNo ratings yet
- Allen Organic Quic RivisionDocument2 pagesAllen Organic Quic Rivisionsaisupreeth0913No ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- Aldehyde and KetoneDocument3 pagesAldehyde and KetoneErica TepepaNo ratings yet
- Reaction of Aldehyde CompleteDocument1 pageReaction of Aldehyde CompleteJoko SusiloNo ratings yet
- Organic Chem NotesDocument49 pagesOrganic Chem NotesPriyaNo ratings yet
- Carbonyl Compounds A-Level NotesDocument4 pagesCarbonyl Compounds A-Level Notesbumblebee9323No ratings yet
- Hydrocarbon: GMP GRDocument30 pagesHydrocarbon: GMP GRVinod AgrawalNo ratings yet
- Reaction SchemeDocument1 pageReaction SchemesakoakimNo ratings yet
- Carbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)Document12 pagesCarbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)agrawaltwinkle2005No ratings yet
- Carbonyl CompoundsDocument10 pagesCarbonyl CompoundsMahendra ChouhanNo ratings yet
- As OrganicDocument7 pagesAs OrganicmalnourishedandstupidNo ratings yet
- GOC Class11thDocument38 pagesGOC Class11thAnju SehrawatNo ratings yet
- Alkynes Medorg2Document6 pagesAlkynes Medorg2AR LazagaNo ratings yet
- Catalyst Note: (PT, Ni, PD)Document8 pagesCatalyst Note: (PT, Ni, PD)Justin Victor AngNo ratings yet
- Phenols and Ethers NotesDocument9 pagesPhenols and Ethers NotesDhanaranjani BNo ratings yet
- Benzene RxnsDocument1 pageBenzene Rxnsapi-465421809No ratings yet
- Alcohols & Ethers TheoryDocument15 pagesAlcohols & Ethers TheorySaif KhanNo ratings yet
- Grignard Reagent C-C Bond: R D O ODocument1 pageGrignard Reagent C-C Bond: R D O OKeshav PargeeNo ratings yet
- Reaction of Carboxylic AcidDocument1 pageReaction of Carboxylic AcidJoko SusiloNo ratings yet
- Table of K ValuesDocument7 pagesTable of K ValuesdasoodaseeNo ratings yet
- AIEEE Chemistry Quick ReviewDocument1 pageAIEEE Chemistry Quick ReviewYashwanth KalyanNo ratings yet
- Flow Chart - HydrocarbonsDocument77 pagesFlow Chart - HydrocarbonsKalyan Reddt100% (2)
- RX Adisi (B)Document29 pagesRX Adisi (B)Wanda RianiNo ratings yet
- Alkene N BenzeneDocument2 pagesAlkene N Benzenemikumo81No ratings yet
- 202003291608409347arun Sethi SteroidDocument7 pages202003291608409347arun Sethi SteroidVishva AegonNo ratings yet
- Flow Chart of Organic Reactions: Substitution (+NHDocument1 pageFlow Chart of Organic Reactions: Substitution (+NHAhhhhhhhhhhhNo ratings yet
- Grignard Reagent C C Bond Mind MapDocument1 pageGrignard Reagent C C Bond Mind MapSii SheikhNo ratings yet
- Pinacol Pinacolone RearrangementDocument3 pagesPinacol Pinacolone RearrangementFahad BashirNo ratings yet
- Organic Chemistry Reaction TableDocument11 pagesOrganic Chemistry Reaction TableKristineNo ratings yet
- 28 HydrocarbonsDocument6 pages28 HydrocarbonsDivyansh SinghNo ratings yet
- MBD Toolkit BDocument1 pageMBD Toolkit BOCRChemistrySaltersNo ratings yet
- Memory Map AromaticsDocument1 pageMemory Map AromaticsOCRChemistrySaltersNo ratings yet
- Medicines by Design..: Revision of Reagents, Conditions and Reaction Types. Click To Go To A Particular SectionDocument39 pagesMedicines by Design..: Revision of Reagents, Conditions and Reaction Types. Click To Go To A Particular SectionOCRChemistrySaltersNo ratings yet
- Is This Your RoomDocument25 pagesIs This Your RoomOCRChemistrySaltersNo ratings yet
- Buffers: Calculating The PH of A Buffer SolutionDocument8 pagesBuffers: Calculating The PH of A Buffer SolutionOCRChemistrySaltersNo ratings yet
- AromaticsDocument1 pageAromaticsOCRChemistrySaltersNo ratings yet
- CC Entropy 1Document10 pagesCC Entropy 1OCRChemistrySaltersNo ratings yet
- Ions in Solids and Solutions.: CI 4.5 and Revision of 5.1Document23 pagesIons in Solids and Solutions.: CI 4.5 and Revision of 5.1OCRChemistrySaltersNo ratings yet
- Energy Changes in SolutionsDocument18 pagesEnergy Changes in SolutionsOCRChemistrySaltersNo ratings yet
- Buffer Solutions: How Do Buffers Work?Document2 pagesBuffer Solutions: How Do Buffers Work?OCRChemistrySaltersNo ratings yet
- CimaDocument11 pagesCimaOCRChemistrySaltersNo ratings yet
- The Size of IonsDocument20 pagesThe Size of IonsOCRChemistrySaltersNo ratings yet
- Acids and Bases TranslationDocument1 pageAcids and Bases TranslationOCRChemistrySaltersNo ratings yet
- JB Chemical Ideas 12point3arenesDocument9 pagesJB Chemical Ideas 12point3arenesOCRChemistrySaltersNo ratings yet
- 6.9 Chemistry of ColourDocument7 pages6.9 Chemistry of ColourOCRChemistrySaltersNo ratings yet
- ChromatographyDocument10 pagesChromatographyjayNo ratings yet
- N (G) + 3H (G) 2Nh (G) H - 92 KJ MolDocument7 pagesN (G) + 3H (G) 2Nh (G) H - 92 KJ MolOCRChemistrySaltersNo ratings yet
- 13.6 Oils and FatsDocument9 pages13.6 Oils and FatsOCRChemistrySaltersNo ratings yet
- ThedblockDocument32 pagesThedblockOCRChemistrySaltersNo ratings yet
- X Marks The Spot SS1Document1 pageX Marks The Spot SS1OCRChemistrySaltersNo ratings yet
- Starter For Lesson 11Document5 pagesStarter For Lesson 11OCRChemistrySaltersNo ratings yet
- Disconnection Approach To Synthesis: Illogical Two Group Disconnections I) 1,2-Dioxygenated PatternDocument27 pagesDisconnection Approach To Synthesis: Illogical Two Group Disconnections I) 1,2-Dioxygenated PatternhinhthoiNo ratings yet
- Chemistry 2 Answers ADocument21 pagesChemistry 2 Answers ARonald McdonaldNo ratings yet
- Alcohol Oxidations: I. Basic PrinciplesDocument14 pagesAlcohol Oxidations: I. Basic PrinciplesTethslynn KanuaNo ratings yet
- CHE2522 Course Outline - 2019-2020 Academic Year-Final 24.07.2020Document5 pagesCHE2522 Course Outline - 2019-2020 Academic Year-Final 24.07.2020Lucy ZuluNo ratings yet
- Introdutory Lectures On Enzymes-1Document13 pagesIntrodutory Lectures On Enzymes-1Elizabeth OmobolanleNo ratings yet
- Wachi1994 PDFDocument6 pagesWachi1994 PDFagus kurniawanNo ratings yet
- Report 1 PhysicalDocument16 pagesReport 1 PhysicalAhmed MasoudNo ratings yet
- AQA A Level Chemistry Unit 2 DefinitionsDocument2 pagesAQA A Level Chemistry Unit 2 DefinitionsMuadh ChatiNo ratings yet
- Heterogenus CatalysisDocument31 pagesHeterogenus CatalysisEdwin FlourenzNo ratings yet
- Evaluated Kinetic Data For Combustion ModelingDocument642 pagesEvaluated Kinetic Data For Combustion ModelingKashaf BakaliNo ratings yet
- RetrosynthesisDocument20 pagesRetrosynthesistamilsam19860% (1)
- 01 Isothermal Reactor DesignDocument38 pages01 Isothermal Reactor DesignLê MinhNo ratings yet
- Stereoselective Reactions of Alkenes: Single Diastereoisomers Pre-Existing StereogenicDocument25 pagesStereoselective Reactions of Alkenes: Single Diastereoisomers Pre-Existing StereogenicSubhabrata MabhaiNo ratings yet
- Chem 282Document3 pagesChem 282UrlaNo ratings yet
- Semester-6 3360503 CRE MCQ KRD PDFDocument9 pagesSemester-6 3360503 CRE MCQ KRD PDFDhruv RanaNo ratings yet
- Balancing Equations: Practice ProblemsDocument10 pagesBalancing Equations: Practice ProblemsMelerose Dela SernaNo ratings yet
- Ireland ModelDocument10 pagesIreland ModelPRANAV SREEKUMARNo ratings yet
- Soalan 6 k2 (Rate of Reaction) KimiaDocument3 pagesSoalan 6 k2 (Rate of Reaction) KimiaNadia AhmadNo ratings yet
- Physics Project Report Class 10Document28 pagesPhysics Project Report Class 10Deepak SinghNo ratings yet
- CV 20181212Document5 pagesCV 20181212Piyasan PraserthdamNo ratings yet
- Week 7 CYJ Steady-State Nonisothermal Reactor Design 1-FullDocument23 pagesWeek 7 CYJ Steady-State Nonisothermal Reactor Design 1-FullElyse Kymberly TeohNo ratings yet
- 07 Raction KineticsDocument43 pages07 Raction KineticsestefanoveiraNo ratings yet
- Important Questions For Class 12 Chemistry Chapter 4 Chemical Kinetics Class 12 Important Questions - Learn CBSEDocument40 pagesImportant Questions For Class 12 Chemistry Chapter 4 Chemical Kinetics Class 12 Important Questions - Learn CBSErithimukulaNo ratings yet
- CHEM3220 - Chapter 5 - 2023-24Document22 pagesCHEM3220 - Chapter 5 - 2023-24am0企鵝No ratings yet
- Chem 215 Myers: The Suzuki ReactionDocument10 pagesChem 215 Myers: The Suzuki ReactionRajathi YadavNo ratings yet
- KINETICSOFEPICHLOROHYDRINDocument16 pagesKINETICSOFEPICHLOROHYDRINMuhammad NomanNo ratings yet
- Sinteza Chimica AdamantanDocument4 pagesSinteza Chimica AdamantanclapadusNo ratings yet