Professional Documents
Culture Documents
SDFGHJ
SDFGHJ
Uploaded by
PRAVIN0 ratings0% found this document useful (0 votes)
3 views6 pagessdfghm,
Original Title
sdfghj
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentsdfghm,
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
3 views6 pagesSDFGHJ
SDFGHJ
Uploaded by
PRAVINsdfghm,
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 6
Fugacity: It is derived from Latin, expressed as
fleetness or escaping tendency. It is used
to study extensively phase and chemical reaction
equilibrium.
G = RT ln f +
- Is an constant depends on temperature and nature
of gas.
fugacity has same units as pressure for an ideal gas.
Fugacity coefficient () :
Fugacity coefficient is defined as the ratio of fugacity
of component to its pressure.
=f/P
- Is the measure of non ideal behavior of the gas.
Activity(a):
It is defined as the fugacity of the existing condition
to the standard state fugacity
a=f/ fo
Activity coefficient :
It measure the extent to which real solution depart from
ideality.
ln=bx
ln=bx
Wohls three suffix equation
Margules equation
Van laar equation
Wilson equation
Non random two liquid (NRTL)equation
Universal quasi chemical (UNIQUAC)equation
Universal functional activity coefficient (UNIFAC)
method
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5822)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
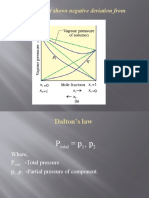
- Where, P - Total Pressure P, P - Partial Pressure of ComponentDocument3 pagesWhere, P - Total Pressure P, P - Partial Pressure of ComponentPRAVINNo ratings yet
- Composition in Gaseous Phase:: y P X P (I 1,2,3 ..N)Document8 pagesComposition in Gaseous Phase:: y P X P (I 1,2,3 ..N)PRAVINNo ratings yet
- Yip=Xiγip (I= 1,2,…..,N) : Where Γi = Activity CoefficientDocument2 pagesYip=Xiγip (I= 1,2,…..,N) : Where Γi = Activity CoefficientPRAVINNo ratings yet
- Where, P - Total Pressure P, P - Partial Pressure of ComponentDocument9 pagesWhere, P - Total Pressure P, P - Partial Pressure of ComponentPRAVINNo ratings yet
- UygfcDocument10 pagesUygfcPRAVINNo ratings yet
- Effect of Pressure On VLE The High Pressure Diagrams Are Above Low PressureDocument7 pagesEffect of Pressure On VLE The High Pressure Diagrams Are Above Low PressurePRAVINNo ratings yet
- Vapiquid IbriumDocument15 pagesVapiquid IbriumPRAVINNo ratings yet
- OIUHGFDXZDocument5 pagesOIUHGFDXZPRAVINNo ratings yet
- Vapiquid IbriumDocument15 pagesVapiquid IbriumPRAVINNo ratings yet
- TFDXZDocument14 pagesTFDXZPRAVINNo ratings yet
- Activity (A) :: It Is Defined As The Fugacity of The Existing Condition To The Standard State FugacityDocument4 pagesActivity (A) :: It Is Defined As The Fugacity of The Existing Condition To The Standard State FugacityPRAVINNo ratings yet
- Activity (A) :: It Is Defined As The Fugacity of The Existing Condition To The Standard State FugacityDocument4 pagesActivity (A) :: It Is Defined As The Fugacity of The Existing Condition To The Standard State FugacityPRAVINNo ratings yet
- PokjhgvcDocument14 pagesPokjhgvcPRAVINNo ratings yet
- Dalton's Law: Where, P - Total Pressure P, P - Partial Pressure of ComponentDocument12 pagesDalton's Law: Where, P - Total Pressure P, P - Partial Pressure of ComponentPRAVINNo ratings yet
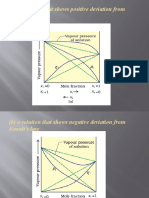
- (A) A Solution That Shows Positive Deviation From Raoult's LawDocument7 pages(A) A Solution That Shows Positive Deviation From Raoult's LawPRAVINNo ratings yet
- Vap EquilibriumDocument14 pagesVap EquilibriumPRAVINNo ratings yet
- Yip=Xiγi P (I= 1,2,…..,N) : Where Γi = Activity CoefficientDocument7 pagesYip=Xiγi P (I= 1,2,…..,N) : Where Γi = Activity CoefficientPRAVINNo ratings yet
- Activity (A) : A F/ F Activity CoefficientDocument3 pagesActivity (A) : A F/ F Activity CoefficientPRAVINNo ratings yet
- Vle 9Document10 pagesVle 9PRAVINNo ratings yet
- (B) A Solution That Shows Negative Deviation From Raoult's LawDocument9 pages(B) A Solution That Shows Negative Deviation From Raoult's LawPRAVINNo ratings yet
- What Is Equilibrium?Document12 pagesWhat Is Equilibrium?PRAVINNo ratings yet
- Vapour LiquidDocument5 pagesVapour LiquidPRAVINNo ratings yet
- Vapour Liquid EquilibriumDocument11 pagesVapour Liquid EquilibriumPRAVINNo ratings yet
- Vle 4Document15 pagesVle 4PRAVINNo ratings yet
- Vapour Liquid EquilibriumDocument15 pagesVapour Liquid EquilibriumPRAVINNo ratings yet