Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
65 viewsIron Carbon Diagram 6
Iron Carbon Diagram 6
Uploaded by
Harris DarThe document discusses the iron-carbon phase diagram, including:
- The phases that exist in iron-carbon alloys at different carbon concentrations and temperatures, such as ferrite, austenite, cementite, and pearlite.
- Key temperatures on the diagram including the eutectoid temperature (723°C) where austenite transforms to pearlite.
- The microstructures of different steel compositions, such as hypoeutectoid steel containing ferrite and pearlite.
- Common applications that take advantage of the different properties imparted by the microstructures, such as pearlite providing strength for cutting tools.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You might also like
- Atm GuideDocument23 pagesAtm GuideGilbert Kamanzi100% (3)
- Diebold Atm Operator ManualDocument72 pagesDiebold Atm Operator ManualHarris Dar50% (2)
- Diebold Atm Operator ManualDocument4 pagesDiebold Atm Operator ManualHarris Dar0% (3)
- Diebold Atm Operator ManualDocument4 pagesDiebold Atm Operator ManualHarris Dar0% (3)
- Refractory PPT IN 150 MW CFBC BOILERDocument25 pagesRefractory PPT IN 150 MW CFBC BOILERkvsagar67% (3)
- The Iron-Iron Carbide (Fe-Fe C) Phase DiagramDocument32 pagesThe Iron-Iron Carbide (Fe-Fe C) Phase DiagramNisaNo ratings yet
- The Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39From EverandThe Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39Rating: 5 out of 5 stars5/5 (1)
- Soil Works For Road ConstructionDocument40 pagesSoil Works For Road ConstructionYaseen YousafNo ratings yet
- The Iron-Iron Carbide Equilibrium DiagramDocument15 pagesThe Iron-Iron Carbide Equilibrium DiagramjhangeerNo ratings yet
- Iron Carbon DiagramDocument23 pagesIron Carbon DiagramdeepakNo ratings yet
- 2 Iron-Carbon Alloy SystemDocument36 pages2 Iron-Carbon Alloy SystemYour EntertainerNo ratings yet
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 pagesSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserNo ratings yet
- Ironiron CarbideequilibriumphasediagramDocument39 pagesIroniron CarbideequilibriumphasediagramSheikh UMARNo ratings yet
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- Iron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Document33 pagesIron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Mahmoud RefaatNo ratings yet
- Introduction-Iron Carbon Phase DiagramDocument31 pagesIntroduction-Iron Carbon Phase DiagramTHE BBEASTNo ratings yet
- The Iron-Carbon Phase DiagramDocument16 pagesThe Iron-Carbon Phase DiagramMeena SivasubramanianNo ratings yet
- 03 - Iron - Iron CarbideDocument35 pages03 - Iron - Iron CarbidebotobotoakbarNo ratings yet
- Lecture 9 - Ferrous AlloysDocument31 pagesLecture 9 - Ferrous Alloysmahmoud foudaNo ratings yet
- Lec 7 Fe C DiagramDocument45 pagesLec 7 Fe C DiagramAdnan MehmoodNo ratings yet
- 3 Iron Carbon DiaDocument21 pages3 Iron Carbon DiaChhavi SharmaNo ratings yet
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed El SayadNo ratings yet
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarNo ratings yet
- Practical 1Document9 pagesPractical 1Sami Onur VuralNo ratings yet
- Plain Iron Carbon SteelsDocument5 pagesPlain Iron Carbon Steelsروشان فاطمة روشانNo ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- Engineering Material-II: Iron Carbide Phase DiagramDocument16 pagesEngineering Material-II: Iron Carbide Phase DiagramAla ZiNo ratings yet
- Lecture 2 Material PDFDocument235 pagesLecture 2 Material PDFdatnguyen789jNo ratings yet
- Iron Iron-Carbide Equilibrium SystemDocument26 pagesIron Iron-Carbide Equilibrium SystemHiral HiraniNo ratings yet
- Fe C Phase DiagramDocument12 pagesFe C Phase DiagramDwi Cahyo NugrohoNo ratings yet
- Heat Treatment - Critical Temperatures - Transformation On Heating / CoolingDocument23 pagesHeat Treatment - Critical Temperatures - Transformation On Heating / Coolingsamurai7_77No ratings yet
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasNo ratings yet
- 06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramDocument42 pages06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramTalaat Ahmed Mohamed El-Benawy100% (2)
- Iron Carbon Note 1 2023Document23 pagesIron Carbon Note 1 2023gerrard samuelNo ratings yet
- Heat Treatment of SteelsDocument41 pagesHeat Treatment of Steelsyaswanth1992No ratings yet
- Iron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018Document30 pagesIron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018prasenjitsayantan100% (1)
- Iron-Carbon Phase DiagramDocument30 pagesIron-Carbon Phase Diagramjunaid hassanNo ratings yet
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongNo ratings yet
- Funamental of MetallurgyDocument235 pagesFunamental of Metallurgysoumyo broto dasNo ratings yet
- SteelDocument4 pagesSteelgovimanoNo ratings yet
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed Karim MohamedNo ratings yet
- Iron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingDocument7 pagesIron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingRajulapati Sunil KumarNo ratings yet
- Lesson 5 - Plain Carbon SteelsDocument4 pagesLesson 5 - Plain Carbon SteelsOwen GichangiNo ratings yet
- Fe CdiagramDocument36 pagesFe CdiagramGeorge SingerNo ratings yet
- Unit 4-1-FerrousDocument78 pagesUnit 4-1-FerrousNisha Jaiswal100% (1)
- EMM LectureDocument38 pagesEMM Lecturelatendra kumar srivastavNo ratings yet
- Fe-Fe3c Diagram VerygoodDocument3 pagesFe-Fe3c Diagram VerygoodRama Krishna Reddy DonthireddyNo ratings yet
- Metastable Iron-Carbon (Fe-C) Phase DiagramDocument3 pagesMetastable Iron-Carbon (Fe-C) Phase DiagramupenderNo ratings yet
- Iron Carbon DiagramDocument8 pagesIron Carbon Diagramashok pradhanNo ratings yet
- FeC and TTT DiagramsDocument12 pagesFeC and TTT DiagramsMohamed El-WakilNo ratings yet
- MT 305 Heat Treatment: TemperingDocument13 pagesMT 305 Heat Treatment: TemperingaarvNo ratings yet
- Introduction To Iron Metallurgy PDFDocument90 pagesIntroduction To Iron Metallurgy PDFDrTrinath TalapaneniNo ratings yet
- The Iron-Carbon Equilibrium Diagram: AbstractDocument4 pagesThe Iron-Carbon Equilibrium Diagram: Abstractleodavid87No ratings yet
- Iron Carbon DiagramDocument43 pagesIron Carbon Diagramhrpatel_165No ratings yet
- IRON - CARBON DiagramDocument15 pagesIRON - CARBON Diagramgadde39100% (2)
- Cast Steel: The Iron-Carbon Equilibrium Diagram: AbstractDocument5 pagesCast Steel: The Iron-Carbon Equilibrium Diagram: Abstractchacha4500No ratings yet
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manNo ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- The Metallurgy of Carbon SteelDocument5 pagesThe Metallurgy of Carbon SteeltsoheilNo ratings yet
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Oxy-Acetylene Welding and Cutting: Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonFrom EverandOxy-Acetylene Welding and Cutting: Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonNo ratings yet
- Blacksmithing on the Farm - With Information on the Materials, Tools and Methods of the BlacksmithFrom EverandBlacksmithing on the Farm - With Information on the Materials, Tools and Methods of the BlacksmithNo ratings yet
- The Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelRating: 5 out of 5 stars5/5 (4)
- Scan ResultsDocument12 pagesScan ResultsHarris DarNo ratings yet
- Elastic and Plastic Deformation: - Metals Deform Both Elastically and PlasticallyDocument24 pagesElastic and Plastic Deformation: - Metals Deform Both Elastically and PlasticallyHarris DarNo ratings yet
- Machine Workshop: What I Have Done in My Visits To WorkshopDocument18 pagesMachine Workshop: What I Have Done in My Visits To WorkshopHarris DarNo ratings yet
- Halliday's Function of Language Theory: Prof. Jamil AkhtarDocument11 pagesHalliday's Function of Language Theory: Prof. Jamil AkhtarHarris DarNo ratings yet
- WhitePaper CheckerDocument9 pagesWhitePaper CheckerHarris DarNo ratings yet
- MoniMax5100T Operator Manual V2Document279 pagesMoniMax5100T Operator Manual V2Harris DarNo ratings yet
- Course Outline BSC Mechanical Eng2015Document10 pagesCourse Outline BSC Mechanical Eng2015Harris DarNo ratings yet
- CS230 Consumables & Spare PartsDocument2 pagesCS230 Consumables & Spare PartsMark FieldsNo ratings yet
- Loose Face Powder With MicrosorbDocument1 pageLoose Face Powder With Microsorbdina cmbeauteNo ratings yet
- 5.standards Gyproc Whitebook 2 MeDocument4 pages5.standards Gyproc Whitebook 2 MeAzhar ShaikhNo ratings yet
- Properties of AlluminiumDocument3 pagesProperties of Alluminiumtapan456No ratings yet
- Polyguard PEDocument2 pagesPolyguard PEabcd1860100% (1)
- Color Coding Chart - AHGDocument3 pagesColor Coding Chart - AHGahmedNo ratings yet
- Mse8 2 PDFDocument27 pagesMse8 2 PDFKrishnanNo ratings yet
- Pipe Thruster 4S GB HK2362 DB 20160912 LowRes 02Document2 pagesPipe Thruster 4S GB HK2362 DB 20160912 LowRes 02CARLOSNo ratings yet
- Addis Ababa University: Faculty of Technology (South)Document51 pagesAddis Ababa University: Faculty of Technology (South)bereketNo ratings yet
- Comparison of The Use of Rutile and Cellulosic Electrodes - TWIDocument11 pagesComparison of The Use of Rutile and Cellulosic Electrodes - TWISumantaNo ratings yet
- RTGH ManualDocument84 pagesRTGH ManualRichardNo ratings yet
- The Benchmark in Plastics ProcessingDocument8 pagesThe Benchmark in Plastics ProcessingvenkithankamNo ratings yet
- BMW GS 90010-1 2020-01 EN (Metallic Coatings and Inorganic Coating Systems)Document12 pagesBMW GS 90010-1 2020-01 EN (Metallic Coatings and Inorganic Coating Systems)Adrian Graciano100% (2)
- High Performance Concrete M85 Mix DesignDocument33 pagesHigh Performance Concrete M85 Mix DesignraguNo ratings yet
- Elements Compounds and Mixtures Study Guide AnswersDocument2 pagesElements Compounds and Mixtures Study Guide AnswersCesar CastilloNo ratings yet
- TDS005-Grade 2 and ASTM A307 BoltingDocument2 pagesTDS005-Grade 2 and ASTM A307 BoltingKrish DoodnauthNo ratings yet
- Suspension and Emulsions - Print - QuizizzDocument4 pagesSuspension and Emulsions - Print - QuizizzLakshmi Kumari100% (1)
- ASTM D 2500-11 - Cloud Point of Petroleum ProductsDocument5 pagesASTM D 2500-11 - Cloud Point of Petroleum ProductsAnak AyamNo ratings yet
- 34 CR Ni Mo 6Document4 pages34 CR Ni Mo 6Jonas AnderssonNo ratings yet
- General Information: Principal Applications Principal ApplicationsDocument8 pagesGeneral Information: Principal Applications Principal ApplicationsrenandNo ratings yet
- Foundry Corporate Presentation 2015 - 55235Document27 pagesFoundry Corporate Presentation 2015 - 55235Denriizkii ArifNo ratings yet
- Dow Solvent Technologies For CO 2 RemovalDocument25 pagesDow Solvent Technologies For CO 2 RemovalWajid NizamiNo ratings yet
- 70Document41 pages70masoud132No ratings yet
- Box Culvert DesignDocument34 pagesBox Culvert DesignMuscadin MakensonNo ratings yet
- Schedule 40 PipeDocument2 pagesSchedule 40 Piperasnowmah2012No ratings yet
- Installing and Shaping Scales On A Small Neck Knife - Nick WheelerDocument35 pagesInstalling and Shaping Scales On A Small Neck Knife - Nick WheelercpNo ratings yet
- Calcium ChlorideDocument17 pagesCalcium ChlorideRobertNo ratings yet
- Bolleballi Naganivrithi (Greendaless) - 1E BSQ 8.4 OnlineDocument2 pagesBolleballi Naganivrithi (Greendaless) - 1E BSQ 8.4 OnlineB.NiviNo ratings yet
Iron Carbon Diagram 6
Iron Carbon Diagram 6
Uploaded by
Harris Dar0 ratings0% found this document useful (0 votes)
65 views15 pagesThe document discusses the iron-carbon phase diagram, including:
- The phases that exist in iron-carbon alloys at different carbon concentrations and temperatures, such as ferrite, austenite, cementite, and pearlite.
- Key temperatures on the diagram including the eutectoid temperature (723°C) where austenite transforms to pearlite.
- The microstructures of different steel compositions, such as hypoeutectoid steel containing ferrite and pearlite.
- Common applications that take advantage of the different properties imparted by the microstructures, such as pearlite providing strength for cutting tools.
Original Description:
Iron Carbon diagram. Industrail Materials.
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses the iron-carbon phase diagram, including:
- The phases that exist in iron-carbon alloys at different carbon concentrations and temperatures, such as ferrite, austenite, cementite, and pearlite.
- Key temperatures on the diagram including the eutectoid temperature (723°C) where austenite transforms to pearlite.
- The microstructures of different steel compositions, such as hypoeutectoid steel containing ferrite and pearlite.
- Common applications that take advantage of the different properties imparted by the microstructures, such as pearlite providing strength for cutting tools.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
65 views15 pagesIron Carbon Diagram 6
Iron Carbon Diagram 6
Uploaded by
Harris DarThe document discusses the iron-carbon phase diagram, including:
- The phases that exist in iron-carbon alloys at different carbon concentrations and temperatures, such as ferrite, austenite, cementite, and pearlite.
- Key temperatures on the diagram including the eutectoid temperature (723°C) where austenite transforms to pearlite.
- The microstructures of different steel compositions, such as hypoeutectoid steel containing ferrite and pearlite.
- Common applications that take advantage of the different properties imparted by the microstructures, such as pearlite providing strength for cutting tools.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 15
Iron Carbon Diagram
Substitutional or Intersitial Sites
When the deviation
from the periodic
arrangement of the
lattice is localized to
the vicinity of only a
few atoms.
It is called a point
defect, or point
imperfection.
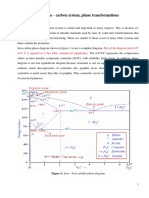
Iron-carbon phase diagram
• Describes the iron-carbon system of alloys
containing up to 6.67% of carbon,
• Discloses the Phases compositions
• Transformations occurring with the alloys
during their cooling or heating.
• Carbon content 6.67% corresponds to the
fixed composition of the iron carbide Fe3C.
Phase compositions of the iron-
carbon alloys at room temperature
• Hypoeutectoid steels (carbon content from 0 to 0.8%)
consist of primary (proeutectoid) ferrite and pearlite.
• Eutectoid steel (carbon content 0.8%) entirely consists
of pearlite.
• Hypereutectoid steels (carbon content from 0.8 to
2.%) consist of primary (proeutectoid)cementite and
pearlite.
• Cast irons (carbon content from 2.0% to 4.3%) consist
of proeutectoid cementite , pearlite and transformed
ledeburite (ledeburite in which austenite transformed
to pearlite).
Critical temperatures In Iron-Carbon
Diagram
• Upper critical temperature (point) A3 is the temperature,
below which ferrite starts to form as a result of ejection
from austenite in the hypoeutectoid alloys.
• Upper critical temperature (point) ACM is the temperature,
below which cementite starts to form as a result of ejection
from austenite in the hypereutectoid alloys.
• Lower critical temperature (point) A1 is the temperature of
the austenite-to-pearlite eutectoid transformation. Below
this temperature austenite does not exist.
• Magnetic transformation temperature A2 is the
temperature below which α-ferrite is
• The following phases are involved in the
transformation, occurring with iron-carbon alloys.
• Liquid
• δ-ferrite
• Austenite
• α-ferrite
• Cementite
• Pearlite.
• L - Liquid solution of carbon in iron;
• δ-ferrite :Solid solution of carbon in iron. The of δ-ferrite is
BCC.
• Austenite : Intersitial solid solutionof carbon in γ-iron.
Austenite has FCC structure, permitting high solubility of
carbon – up to 2% at 1147 ºC.
• α-ferrite – Intersitial solid solution of carbon in α-iron.
ferrite has BCC crystal structure and low solubility of carbon
up to 0.025% at 723ºC. Ferrite exists at room temperature.
• Cementite – iron carbide, intermetallic compound, having
fixed composition Fe3C.
Pearlite
• It is a mixture of two phases. Ferrite and
cementite.
• Lamellar structure composed of alternating
layers of alpha-Iron and cementite.
• The eutectoid composition of austenite is
approximately 0.8% cabon.
• Steel with less carbon content will contain a
ferrite (pro eutectiod) and Pearlite
• Steels with higher carbon contents contains
cementite (Pro eutectoid) .
• The volume fraction of pro eutectoid ferrite
and cementite can be calculated from the
iron/iron—carbide equilibrium phase diagram
using the lever rule.
• Steels with pearlitic (eutectoid composition)
or near-pearlitic microstructure (near-
eutectoid composition) can be drawn into thin
wires.
• Such wires, commercially used as piano wires
• Often bundled into ropes,for suspension
bridges,
• As steel cord for tire reinforcement.
• Eutectoid steel can in principle be transformed
completely into pearlite.
• Hypoeutectoid steels can also be completely
pearlitic if transformed at a temperature below
the normal eutectoid.
• Pearlite can be hard and strong but is not
particularly tough.
• It can be wear resistant because of a strong
lamellar network of ferrite and cementite.
• Examples of applications include cutting tools,
high strength wires, knives, chisels, and nails.
•At this temperature
•The tie line shows that
the alpha phase contains
5.2%B
•The liquid phase contains
34.5%B.
•Fraction of alpha = (34.5 - 23.7)
/ (34.5 - 5.2) = 0.3686
•The percentage of alpha
present can be calculated =
0.3686 x 100 = 36.86%
•The alpha and the liquid make
up 100% of the alloy's
composition:
•Percentage of liquid = 100 -
36.86 = 63.14%
You might also like
- Atm GuideDocument23 pagesAtm GuideGilbert Kamanzi100% (3)
- Diebold Atm Operator ManualDocument72 pagesDiebold Atm Operator ManualHarris Dar50% (2)
- Diebold Atm Operator ManualDocument4 pagesDiebold Atm Operator ManualHarris Dar0% (3)
- Diebold Atm Operator ManualDocument4 pagesDiebold Atm Operator ManualHarris Dar0% (3)
- Refractory PPT IN 150 MW CFBC BOILERDocument25 pagesRefractory PPT IN 150 MW CFBC BOILERkvsagar67% (3)
- The Iron-Iron Carbide (Fe-Fe C) Phase DiagramDocument32 pagesThe Iron-Iron Carbide (Fe-Fe C) Phase DiagramNisaNo ratings yet
- The Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39From EverandThe Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 21 to EN 39Rating: 5 out of 5 stars5/5 (1)
- Soil Works For Road ConstructionDocument40 pagesSoil Works For Road ConstructionYaseen YousafNo ratings yet
- The Iron-Iron Carbide Equilibrium DiagramDocument15 pagesThe Iron-Iron Carbide Equilibrium DiagramjhangeerNo ratings yet
- Iron Carbon DiagramDocument23 pagesIron Carbon DiagramdeepakNo ratings yet
- 2 Iron-Carbon Alloy SystemDocument36 pages2 Iron-Carbon Alloy SystemYour EntertainerNo ratings yet
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 pagesSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserNo ratings yet
- Ironiron CarbideequilibriumphasediagramDocument39 pagesIroniron CarbideequilibriumphasediagramSheikh UMARNo ratings yet
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- Iron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Document33 pagesIron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Mahmoud RefaatNo ratings yet
- Introduction-Iron Carbon Phase DiagramDocument31 pagesIntroduction-Iron Carbon Phase DiagramTHE BBEASTNo ratings yet
- The Iron-Carbon Phase DiagramDocument16 pagesThe Iron-Carbon Phase DiagramMeena SivasubramanianNo ratings yet
- 03 - Iron - Iron CarbideDocument35 pages03 - Iron - Iron CarbidebotobotoakbarNo ratings yet
- Lecture 9 - Ferrous AlloysDocument31 pagesLecture 9 - Ferrous Alloysmahmoud foudaNo ratings yet
- Lec 7 Fe C DiagramDocument45 pagesLec 7 Fe C DiagramAdnan MehmoodNo ratings yet
- 3 Iron Carbon DiaDocument21 pages3 Iron Carbon DiaChhavi SharmaNo ratings yet
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed El SayadNo ratings yet
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarNo ratings yet
- Practical 1Document9 pagesPractical 1Sami Onur VuralNo ratings yet
- Plain Iron Carbon SteelsDocument5 pagesPlain Iron Carbon Steelsروشان فاطمة روشانNo ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- Engineering Material-II: Iron Carbide Phase DiagramDocument16 pagesEngineering Material-II: Iron Carbide Phase DiagramAla ZiNo ratings yet
- Lecture 2 Material PDFDocument235 pagesLecture 2 Material PDFdatnguyen789jNo ratings yet
- Iron Iron-Carbide Equilibrium SystemDocument26 pagesIron Iron-Carbide Equilibrium SystemHiral HiraniNo ratings yet
- Fe C Phase DiagramDocument12 pagesFe C Phase DiagramDwi Cahyo NugrohoNo ratings yet
- Heat Treatment - Critical Temperatures - Transformation On Heating / CoolingDocument23 pagesHeat Treatment - Critical Temperatures - Transformation On Heating / Coolingsamurai7_77No ratings yet
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasNo ratings yet
- 06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramDocument42 pages06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramTalaat Ahmed Mohamed El-Benawy100% (2)
- Iron Carbon Note 1 2023Document23 pagesIron Carbon Note 1 2023gerrard samuelNo ratings yet
- Heat Treatment of SteelsDocument41 pagesHeat Treatment of Steelsyaswanth1992No ratings yet
- Iron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018Document30 pagesIron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018prasenjitsayantan100% (1)
- Iron-Carbon Phase DiagramDocument30 pagesIron-Carbon Phase Diagramjunaid hassanNo ratings yet
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongNo ratings yet
- Funamental of MetallurgyDocument235 pagesFunamental of Metallurgysoumyo broto dasNo ratings yet
- SteelDocument4 pagesSteelgovimanoNo ratings yet
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed Karim MohamedNo ratings yet
- Iron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingDocument7 pagesIron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingRajulapati Sunil KumarNo ratings yet
- Lesson 5 - Plain Carbon SteelsDocument4 pagesLesson 5 - Plain Carbon SteelsOwen GichangiNo ratings yet
- Fe CdiagramDocument36 pagesFe CdiagramGeorge SingerNo ratings yet
- Unit 4-1-FerrousDocument78 pagesUnit 4-1-FerrousNisha Jaiswal100% (1)
- EMM LectureDocument38 pagesEMM Lecturelatendra kumar srivastavNo ratings yet
- Fe-Fe3c Diagram VerygoodDocument3 pagesFe-Fe3c Diagram VerygoodRama Krishna Reddy DonthireddyNo ratings yet
- Metastable Iron-Carbon (Fe-C) Phase DiagramDocument3 pagesMetastable Iron-Carbon (Fe-C) Phase DiagramupenderNo ratings yet
- Iron Carbon DiagramDocument8 pagesIron Carbon Diagramashok pradhanNo ratings yet
- FeC and TTT DiagramsDocument12 pagesFeC and TTT DiagramsMohamed El-WakilNo ratings yet
- MT 305 Heat Treatment: TemperingDocument13 pagesMT 305 Heat Treatment: TemperingaarvNo ratings yet
- Introduction To Iron Metallurgy PDFDocument90 pagesIntroduction To Iron Metallurgy PDFDrTrinath TalapaneniNo ratings yet
- The Iron-Carbon Equilibrium Diagram: AbstractDocument4 pagesThe Iron-Carbon Equilibrium Diagram: Abstractleodavid87No ratings yet
- Iron Carbon DiagramDocument43 pagesIron Carbon Diagramhrpatel_165No ratings yet
- IRON - CARBON DiagramDocument15 pagesIRON - CARBON Diagramgadde39100% (2)
- Cast Steel: The Iron-Carbon Equilibrium Diagram: AbstractDocument5 pagesCast Steel: The Iron-Carbon Equilibrium Diagram: Abstractchacha4500No ratings yet
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manNo ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- The Metallurgy of Carbon SteelDocument5 pagesThe Metallurgy of Carbon SteeltsoheilNo ratings yet
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Oxy-Acetylene Welding and Cutting: Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonFrom EverandOxy-Acetylene Welding and Cutting: Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonNo ratings yet
- Blacksmithing on the Farm - With Information on the Materials, Tools and Methods of the BlacksmithFrom EverandBlacksmithing on the Farm - With Information on the Materials, Tools and Methods of the BlacksmithNo ratings yet
- The Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelRating: 5 out of 5 stars5/5 (4)
- Scan ResultsDocument12 pagesScan ResultsHarris DarNo ratings yet
- Elastic and Plastic Deformation: - Metals Deform Both Elastically and PlasticallyDocument24 pagesElastic and Plastic Deformation: - Metals Deform Both Elastically and PlasticallyHarris DarNo ratings yet
- Machine Workshop: What I Have Done in My Visits To WorkshopDocument18 pagesMachine Workshop: What I Have Done in My Visits To WorkshopHarris DarNo ratings yet
- Halliday's Function of Language Theory: Prof. Jamil AkhtarDocument11 pagesHalliday's Function of Language Theory: Prof. Jamil AkhtarHarris DarNo ratings yet
- WhitePaper CheckerDocument9 pagesWhitePaper CheckerHarris DarNo ratings yet
- MoniMax5100T Operator Manual V2Document279 pagesMoniMax5100T Operator Manual V2Harris DarNo ratings yet
- Course Outline BSC Mechanical Eng2015Document10 pagesCourse Outline BSC Mechanical Eng2015Harris DarNo ratings yet
- CS230 Consumables & Spare PartsDocument2 pagesCS230 Consumables & Spare PartsMark FieldsNo ratings yet
- Loose Face Powder With MicrosorbDocument1 pageLoose Face Powder With Microsorbdina cmbeauteNo ratings yet
- 5.standards Gyproc Whitebook 2 MeDocument4 pages5.standards Gyproc Whitebook 2 MeAzhar ShaikhNo ratings yet
- Properties of AlluminiumDocument3 pagesProperties of Alluminiumtapan456No ratings yet
- Polyguard PEDocument2 pagesPolyguard PEabcd1860100% (1)
- Color Coding Chart - AHGDocument3 pagesColor Coding Chart - AHGahmedNo ratings yet
- Mse8 2 PDFDocument27 pagesMse8 2 PDFKrishnanNo ratings yet
- Pipe Thruster 4S GB HK2362 DB 20160912 LowRes 02Document2 pagesPipe Thruster 4S GB HK2362 DB 20160912 LowRes 02CARLOSNo ratings yet
- Addis Ababa University: Faculty of Technology (South)Document51 pagesAddis Ababa University: Faculty of Technology (South)bereketNo ratings yet
- Comparison of The Use of Rutile and Cellulosic Electrodes - TWIDocument11 pagesComparison of The Use of Rutile and Cellulosic Electrodes - TWISumantaNo ratings yet
- RTGH ManualDocument84 pagesRTGH ManualRichardNo ratings yet
- The Benchmark in Plastics ProcessingDocument8 pagesThe Benchmark in Plastics ProcessingvenkithankamNo ratings yet
- BMW GS 90010-1 2020-01 EN (Metallic Coatings and Inorganic Coating Systems)Document12 pagesBMW GS 90010-1 2020-01 EN (Metallic Coatings and Inorganic Coating Systems)Adrian Graciano100% (2)
- High Performance Concrete M85 Mix DesignDocument33 pagesHigh Performance Concrete M85 Mix DesignraguNo ratings yet
- Elements Compounds and Mixtures Study Guide AnswersDocument2 pagesElements Compounds and Mixtures Study Guide AnswersCesar CastilloNo ratings yet
- TDS005-Grade 2 and ASTM A307 BoltingDocument2 pagesTDS005-Grade 2 and ASTM A307 BoltingKrish DoodnauthNo ratings yet
- Suspension and Emulsions - Print - QuizizzDocument4 pagesSuspension and Emulsions - Print - QuizizzLakshmi Kumari100% (1)
- ASTM D 2500-11 - Cloud Point of Petroleum ProductsDocument5 pagesASTM D 2500-11 - Cloud Point of Petroleum ProductsAnak AyamNo ratings yet
- 34 CR Ni Mo 6Document4 pages34 CR Ni Mo 6Jonas AnderssonNo ratings yet
- General Information: Principal Applications Principal ApplicationsDocument8 pagesGeneral Information: Principal Applications Principal ApplicationsrenandNo ratings yet
- Foundry Corporate Presentation 2015 - 55235Document27 pagesFoundry Corporate Presentation 2015 - 55235Denriizkii ArifNo ratings yet
- Dow Solvent Technologies For CO 2 RemovalDocument25 pagesDow Solvent Technologies For CO 2 RemovalWajid NizamiNo ratings yet
- 70Document41 pages70masoud132No ratings yet
- Box Culvert DesignDocument34 pagesBox Culvert DesignMuscadin MakensonNo ratings yet
- Schedule 40 PipeDocument2 pagesSchedule 40 Piperasnowmah2012No ratings yet
- Installing and Shaping Scales On A Small Neck Knife - Nick WheelerDocument35 pagesInstalling and Shaping Scales On A Small Neck Knife - Nick WheelercpNo ratings yet
- Calcium ChlorideDocument17 pagesCalcium ChlorideRobertNo ratings yet
- Bolleballi Naganivrithi (Greendaless) - 1E BSQ 8.4 OnlineDocument2 pagesBolleballi Naganivrithi (Greendaless) - 1E BSQ 8.4 OnlineB.NiviNo ratings yet