Professional Documents
Culture Documents
Carcinogenesis Patho-1
Carcinogenesis Patho-1
Uploaded by
Alishba Mushtaq0 ratings0% found this document useful (0 votes)
7 views33 pages1. Carcinogenesis is a multistep process involving genetic damage from environmental and hereditary factors that transform normal cells into malignant ones.
2. Key targets of genetic damage are proto-oncogenes, tumor suppressor genes, genes regulating apoptosis, and DNA repair genes. Damage to these genes can result in uncontrolled cell growth and the development of cancer.
3. Environmental carcinogens like chemicals, radiation, and infections can cause genetic mutations through direct DNA damage or metabolic activation. Inherited gene mutations also increase cancer risk. Most transformed cells die but some may form malignant clones through clonal expansion.
Original Description:
carcino
Original Title
2. Carcinogenesis Patho-1
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1. Carcinogenesis is a multistep process involving genetic damage from environmental and hereditary factors that transform normal cells into malignant ones.
2. Key targets of genetic damage are proto-oncogenes, tumor suppressor genes, genes regulating apoptosis, and DNA repair genes. Damage to these genes can result in uncontrolled cell growth and the development of cancer.
3. Environmental carcinogens like chemicals, radiation, and infections can cause genetic mutations through direct DNA damage or metabolic activation. Inherited gene mutations also increase cancer risk. Most transformed cells die but some may form malignant clones through clonal expansion.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
7 views33 pagesCarcinogenesis Patho-1
Carcinogenesis Patho-1
Uploaded by
Alishba Mushtaq1. Carcinogenesis is a multistep process involving genetic damage from environmental and hereditary factors that transform normal cells into malignant ones.
2. Key targets of genetic damage are proto-oncogenes, tumor suppressor genes, genes regulating apoptosis, and DNA repair genes. Damage to these genes can result in uncontrolled cell growth and the development of cancer.
3. Environmental carcinogens like chemicals, radiation, and infections can cause genetic mutations through direct DNA damage or metabolic activation. Inherited gene mutations also increase cancer risk. Most transformed cells die but some may form malignant clones through clonal expansion.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 33
Neoplasia: Carcinogenesis
Ayesha Sana (lecturer)
Epidemiology
• Increasing / decreasing incidence
•Environmental and Geographic causes
•Death from Ca breast 4-5 fold higher in USA and Ca sto
mach 7 fold higher in japan
•Ca liver infrequent in USA but most lethal in Africa
•Exposure to sun light .
•Different cancer have different incidences in male and f
emales
•
Epidemiology
• Extremes of age. Increasing age after 55-75. old
er persons have a greater propensity to develo
p neoplasms from lack of effective control mec
hanisms, accumulation of somatic mutations, d
ecline in immune competency. Cause of death i
n 10% children under 15 yrs
• Personal practices: diet -cigarette smoke - lung
cancer. Alcohol. Sexual practices
• Illegal, immoral, fattening - carcinogenic
Occupational cancers
• carcinogenic Chemicals - man-made natural compounds (afl
atoxins - liver cancer)
• Aniline dyes - bladder ca. drugs
• Arsenic: metal smelting, medications –lung, skin ca
• Asbestos: roofing paper, floor tiles – lung git
• Benzene: printing, rubber, paint, dry cleaning -leukemia
• Beryllium: missile feul - lung
• Cadmium: plating, batteries - prostate
• Chromium: paints, pigments, preservative
• Ethylene oxide: ripening agent foods, textile- leukemia
• Nickel: plating, batteries, ceramics – lung nose
• Radon: decay of uranium containing minerals - lung
• Vinyl chloride: refrigerant, adhesive for plastic -liver
Hereditary causes
• Genomic Instability
• Inherited defects in DNA repair genes. 5-10% tumors.
Impact of heredity is subtle or indirect. Genotype may inf
luence the likelihood of environmentally induced tumors
• 3 categories
• Autosomal recessive syndrome of defective DNA repa
ir. HNPCC syndrome (Hereditary non polyposis colon ca),
Xeroderma pigmentosum, defects in homologus recombi
nation DNA repair system (bloom syndrome)
Hereditary factors
• Autosomal dominant cancer syndromes:
• Inheritance of single mutated gene
• Chromosomes which have absent or defective anti-onco
genes that control growth. 40%Retinoblastomas familial,
Germ line mutation of tumor suppressor genes
• Associated with specific phenotype marker
• Familial cancers of uncertain origin
• Pattern of inheritance unclear. Ca colon, breast, ovary, b
rain.
• Early age at onset, two or more close relatives effected,
multiple or bilateral. Not associated with specific marker
phenotypes
Etiology of cancer
Carcinogenic agents
• Three classes
• Chemical
• Radiation
• microbial
• Many agents may act in concert to produce m
ultiple genetic abnormalities
Chemical carcinogens
• Direct acting & Indirect acting
• Direct acting
• Require no metabolic conversion
• Generally weak carcinogens
– Alkylating agents:
• β propiolactone,
• dimethyl sulphate,
• anticancer drugs like chlorambucil Cyclophosph
amide
– Acylating agents:
• Diethyl carbamyl chloride
• 1-acetyl imidazole
Chemical carcinogens
• Indirect acting: Require metabolic conversion. Enzymati
c pathways involved e.g. P-450-dependent monooxygena
se. Polymorphic enzyme, Specific allele of enzyme inherit
ed
• Polycyclic and heterocyclic aromatic hydrocarbons: tobac
co combustion, fossil feuls, smoked meat and fish and an
imal fat in broiling meat. HC form addition products with
cellular molecules.
• Benz(a) anthracene, Benzo(a)pyrene (tobacco)
• Aromatic amines, amides, azo dyes: (food &colors)
• 2-Naphthylamine, Benzidine
• Acetyl amino fluorine
• Dimethylaminoazobenzene
Chemical carcinogens
• Indirect acting
• Natural plant and microbial products:
• Aflatoxin B1 (Aspergillus)
• Griseofulvin
• Betel nuts
• Safrole
• Others:
• Nitrosamines (pickled food) and amides
• Vinyl chloride, nickel, chromium
• Insecticides, fungicides
• Polychlorinated biphenyls
• Nitrites food preservative
Chemical carcinogenesis
Mechanism of action:
Mutagenic. Contain highly reactive electrophile
groups that form chemical adducts with DNA,
RNA and proteins. Any gene can be target but
commonly mutation in RAS & TP53 genes
• There are two steps:
• Initiation and Promotion
Chemical carcinogenesis Initiation
An initiating carcinogenic agent has highly reacti
ve electrophile groups that irreversibly damages c
ell DNA - mutagenic. E.g. alkylating agents like c
yclophosphamide, polycyclic aromatic hydrocarb
ons like epoxides found in smoked foods, aromati
c amines or azo dyes used in food & coloring, Afl
atoxins in moldy peanuts, nitrosamines in pickled
foods.
Chemical carcinogenesis Promotion
Initiator is followed by promoter which acts (reve
rsibly) to cause proliferation of neoplastic cell clo
ne. There appears to be "dose-threshold" concentr
ation of promoter below which neoplasia will not
occur. Not mutagenic
Examples: phenols, hormones (Estrogen), drugs (
diethyl-stilbesterol, chemicals (cyclamates used a
s sweeteners).
Microbial carcinogenesis
• Oncogenic DNA Viruses:
– Hepatitis B virus Hepatocellular carcinomas. Immunologi
cally mediated chronic Inflammation, stimulation of hep
atocyte cell proliferation, production of reactive Oxygen
species
– Epstein Barr virus EBV African Burkitt's lymphoma, Hodg
kins lymphoma, nasopharyngeal ca
– Human papilloma virus HPV – E 6& 7 proteins bind to p5
3 and RB. Squamous cell carcinomas of cervix and anoge
nital squamous papillomas
– Kaposi sarcoma herpesvirus KSHV (HHV8)
Microbial carcinogenesis
• Oncogenic RNA V:
– Human T cell lymphotropic virus HTLV-1
– Hepatitis C virus Hepatocellular carcinomas
Microbial carcinogenesis
• Helicobactor pylori:
• Immunologically mediated chronic Inflammati
on, stimulation of gastric cell proliferation, pro
duction of reactive Oxygen species
• Cag A genes
• gastric Adenocarcinoma, MALT lymphoma
Radiation carcinogenesis
• Sun light - Ultraviolet light - induces pyrimidine dimers i
n DNA. Squamous cell ca, melanoma, basal cell ca
• Ionizing radiation – chromosome break, translocation, i
nduces mutations in DNA.
• Gamma radiation - leukemia, thyroid, lung, colon, and b
reast cancers.
• X-rays – melanoma, squamous cell ca skin
• Nuclear fission – leukemia, thyroid, breast, colon and lu
ng ca
Radiation carcinogenesis
• Radiation, either as low level long-term environmental
gamma rays or as higher dose therapeutic radiation, ca
n also produce genetic mutations, Chromosome breaka
ge, translocations, point mutations, Double stranded D
NA breaks,
• Australia, new zealand greater sunlight. induces pyrimid
ine dimers in DNA, repaired by nucleotide excision repa
ir pathway. Excessive exposure, overwhelms repair syste
ms. Xeroderma pigmentosum
• Sun bathing - melanoma
Carcinogenesis: Molecular basis
• Fundamental principles
• Non lethal genetic damage /mutation
• due to
– Environmental agents radiation, chemical, virus
– Inherited in germ line
• tumors arise from clonal expansion of single progenito
r cell that have incurred genetic damage
Molecular basis of carcinogenesis
• Principal target are four classes of regulatory genes.
– Genes promoting cell growth proto-oncogenes
– Genes inhibiting cell growth tumor suppressor ge
nes. (recessive)
– Genes regulating apoptosis (dominant)
– Genes regulating DNA repair
Molecular basis of carcinogenesis
• Proto-oncogenes: normal cellular genes whose products
promote cell proliferation.
Oncogenes: mutant alleles of proto-oncogenes that funct
ion autonomously without a requirement for normal gro
wth-promoting signals. (dominant). When expressed the
y induce a transformed phenotype in cells
• Tumor suppressor genes: prevent uncontrolled growth. If
damaged, allow transformed phenotype to develop.
• Both alleles must be damaged for transformation to occu
r. Haploinsuffuiciency in some cases. Two groups
• Governors: RB mutation removes break on cellular prolif
eration
• Guardians: TP53 sense genomic damage. Either stop proli
feration or induce apoptosis. Loss permits mutations in g
enes – mutator phenotype
Molecular basis of carcinogenesis
– Genes regulating apoptosis (dominant)
– Genes regulating DNA repair
• Both may act like proto-oncogenes or tumor s
uppressor genes
Carcinogenesis: Molecular basis
• Genetic damage with DNA alterations leads to point m
utations of genes, translocations of genetic material be
tween chromosomes and gene reduplication with ampl
ification. These alterations transform proto-oncogenes
into oncogenes.
• The proto-oncogenes may play a role in growth promot
ion and regulation in normal cells, perhaps in embryog
enesis, but are typically "turned off" in adults. They are
"turned on" by transformation
Cellular Transformation
•Some factor causes a cell to be transformed to a neoplasti
c cell that is not controlled by normal body processes.
•Probably most transformed cells die because they are too
abnormal to function or are abnormal enough for the body'
s immune system to destroy them.
•If the factors promoting neoplasia persist, a transformed c
ell may some day give rise to a clone that does continue to
grow.
•Some neoplastic growth is influenced by host factors. Est
rogenic hormones aid growth of breast fibroadenomas or c
arcinomas and uterine leiomyomas because the tumor cells
have hormone receptors.
Clonality
Neoplastic cells tend to be monoclonal, or similar in ge
netic makeup, indicating origin from a transformed cel
l. Non-neoplastic proliferations (reactions to inflammat
ion) have cells that are polyclonal in origin.
The concept of "tumor progression" holds that subclon
es may arise over time from the original malignant clo
ne. Subclones may differ from original clone in charac
teristics such as invasiveness, metastatic potential, and
response to therapy.
Characteristics of
Transformed (Neoplastic) Cells
• Growth not inhibited by contact with surrounding cell
s and is not dependent on anchorage to solid surface.
• may attain immortality / ability to divide indefinitely
• Are discohesive and transplantable-favoring invasion
and metastasis.
• Can bind to laminin and fibronectin in connective tiss
ues, secrete collagenases or proteases and invade.
Tumor Genetics
Neoplasms have a greater tendency to
• Point mutations activate / inactivate gene product
• Karyotypic abnormalities such as translocations, d
eletions. Leukemias and lymphomas are famous for t
his. Philadelphia (Ph1) chromosome of chronic myel
ogenous leukemia
•t(8:14) translocation in Burkitt's lymphomas.
• gene amplifications (activators of proto-oncogenes
). Neuroblastoma and breast ca
•Aneuploidy: number of chromosoes not multiple of
23
Epigenetic modifications
• Reversible heritble changes in gene expression t
hat occur without mutations.
• DNA methylation, post translational modificatio
n of histones. Gene silencing
• Selective Hypermethylation and hypomethylatio
nin cancer cells
Micro RNAs (MiRNA) or oncomirs
• Non coding SS, 22 nucleotides
• Negative regulator genes. Inhibit gene expression pos
t transcriptionally by repressing translation or mRNA
cleavage.
• Increase expression of RAS ,MYC oncogenes or reduci
ng expression of tumor suppression genes
• Control cell growth, differentiation, survival.
Carcinogenesis multi-step process
at genetic and phenotypic levels
• Mutation in no single gene is sufficient to cause cancer
• Phenotypic attributes characteristic of malignancy develop
when multiple mutations involving multiple genes accumul
ate.
• Cancers can arise from non-neoplastic precursor lesions
• Tumor progression: The stepwise accumulation of mutation
s and increasing malignancy.
• Phenotypic attributes include excessive growth, local invasi
veness, ability to form metastases
Carcinogenesis multi-step process
at genetic and phenotypic levels
• Multiple mutations in different cells result in heterogenei
ty – subclones with different characteristics - growth rate
, invasiveness, drug susceptibility, Antigenecity
• Immune and non immune selection pressures. Highly ant
igenic are destroyed by immune system. Tumors with red
uced growth factor requirements are selected
• Tumor that recur after chemotherapy are almost resistan
t to drug
• So genetic evolution and selection explains the propertie
s of cancers
You might also like
- Veterinary OncologyDocument311 pagesVeterinary Oncologyzoran gacevski100% (4)
- Molecular Basis of CancerDocument35 pagesMolecular Basis of CancerimmortalneoNo ratings yet
- Etiology If NeoplasmDocument18 pagesEtiology If NeoplasmSiraj AnsariNo ratings yet
- Cancer: Etiologic Agents and General MechanismsDocument60 pagesCancer: Etiologic Agents and General MechanismsjithinnxNo ratings yet
- Biochemistry of CancerDocument30 pagesBiochemistry of Cancersafalife11No ratings yet
- Oncology Nursing Oncology What Is Cancer? International IncidenceDocument10 pagesOncology Nursing Oncology What Is Cancer? International IncidenceNeweeJoonYowNo ratings yet
- Oncology Nursing Oncology What Is Cancer?Document10 pagesOncology Nursing Oncology What Is Cancer?NeweeJoonYow100% (1)
- Cancer Genetics: Dr. Zeyad Akawi Jreisat, M.D., M.A., PH.DDocument44 pagesCancer Genetics: Dr. Zeyad Akawi Jreisat, M.D., M.A., PH.DAzry MustapaNo ratings yet
- Cancer: Etiologic Agents and General Mechanisms: Salvador J. Diaz-Cano S.j.diaz-Cano@qmul - Ac.ukDocument60 pagesCancer: Etiologic Agents and General Mechanisms: Salvador J. Diaz-Cano S.j.diaz-Cano@qmul - Ac.ukSi RiNo ratings yet
- Carcinogenesis and Mutagenesis - ConvertedDocument16 pagesCarcinogenesis and Mutagenesis - Convertedam55eer55No ratings yet
- Biology, Clinical Manifestations, and Treatment of CancerDocument48 pagesBiology, Clinical Manifestations, and Treatment of CancerNita AndiniNo ratings yet
- Principle of OncologyDocument79 pagesPrinciple of OncologyJoyatideb SinhaNo ratings yet
- Cancer 3 - 2022Document26 pagesCancer 3 - 2022Joshua KaoNo ratings yet
- 5 Molecular Basis of CancerDocument46 pages5 Molecular Basis of Cancer202210034No ratings yet
- Cellular AberrationsDocument37 pagesCellular AberrationsJess NinaNo ratings yet
- Cancer Pathophysiology: Radhika D Prabhu MS124129Document43 pagesCancer Pathophysiology: Radhika D Prabhu MS124129Radhika PrabhuNo ratings yet
- Cancer Pathophysiology 4th SemDocument43 pagesCancer Pathophysiology 4th SemRadhika PrabhuNo ratings yet
- Pokok Bahasan Nutrasetikal Ke 3Document69 pagesPokok Bahasan Nutrasetikal Ke 3Elsha CantikaNo ratings yet
- Week 7 CancerDocument32 pagesWeek 7 CancertahaberrkayNo ratings yet
- Biology, Clinical Manifestations, and Treatment of Cancer: Unit III: Cell Proliferation: CancerDocument48 pagesBiology, Clinical Manifestations, and Treatment of Cancer: Unit III: Cell Proliferation: CancerShruthi Y nursingNo ratings yet
- Genetics of CancerDocument64 pagesGenetics of CancerErin HillNo ratings yet
- CLIENTS WITH PROBLEMS IN CELLULAR ABERRATIONS Notes For StudentsDocument39 pagesCLIENTS WITH PROBLEMS IN CELLULAR ABERRATIONS Notes For StudentsAno NymousNo ratings yet
- Carcinogenesis: Hematology-Oncology Division Child Health Dept. University of Sumatera UtaraDocument13 pagesCarcinogenesis: Hematology-Oncology Division Child Health Dept. University of Sumatera UtaraAhmed MawardiNo ratings yet
- Neoplastic Diseases: P. Manyau School of Pharmacy University of ZimbabweDocument35 pagesNeoplastic Diseases: P. Manyau School of Pharmacy University of Zimbabwebrain bareNo ratings yet
- Cancer-Latest Trends in DiagnosisDocument58 pagesCancer-Latest Trends in DiagnosisTomson ThomasNo ratings yet
- Neoplasia: Dr. Farah ZahidDocument31 pagesNeoplasia: Dr. Farah ZahidFarah ZahidNo ratings yet
- Carcinogen Is IsDocument48 pagesCarcinogen Is IsAbirami SankarNo ratings yet
- CancerDocument24 pagesCancerarshadmuhammedNo ratings yet
- Oncology Nursing DR AmelDocument103 pagesOncology Nursing DR Amelhalaladham666No ratings yet
- Preparing For Cytotoxic ProductionDocument160 pagesPreparing For Cytotoxic ProductionThe ScienceNo ratings yet
- Molecular Basis of CancerDocument41 pagesMolecular Basis of Cancer페 리알No ratings yet
- Genetic DisordersDocument30 pagesGenetic DisordersNikoh Anthony EwayanNo ratings yet
- Oncology NursingDocument10 pagesOncology NursingCham Rafaela Conese100% (3)
- Cancer NursingDocument132 pagesCancer NursingKenneth Manalang100% (1)
- Carcinogenesis: Tee L. GuidottiDocument21 pagesCarcinogenesis: Tee L. GuidottiLhyne PablicoNo ratings yet
- BM 8-9 OncogenesisDocument139 pagesBM 8-9 OncogenesisabdullahshiddiqadamNo ratings yet
- Cellular Abberation 1Document35 pagesCellular Abberation 1MacaroonsNo ratings yet
- The Genetics of CancerDocument36 pagesThe Genetics of CancerRAFIEYANNo ratings yet
- Oncologic NursingDocument132 pagesOncologic Nursingɹǝʍdןnos100% (10)
- Kuliah Iii Patologi Anatomi Blok OnkologiDocument39 pagesKuliah Iii Patologi Anatomi Blok OnkologiandreNo ratings yet
- Genetics - Molecular Basis of CancerDocument53 pagesGenetics - Molecular Basis of CancerTataning RahayuNo ratings yet
- Basis of Cancer 1Document62 pagesBasis of Cancer 1RuthNo ratings yet
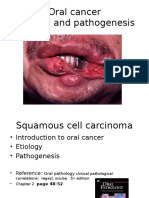
- Oral Cancer Etiology and PathogenesisDocument12 pagesOral Cancer Etiology and PathogenesisBibek RajNo ratings yet
- BB5506: The Biology, Genetics and Treatment of Human CancerDocument54 pagesBB5506: The Biology, Genetics and Treatment of Human Cancerpriyaa100% (1)
- Principles of Neoplasia (New)Document125 pagesPrinciples of Neoplasia (New)egyfellowpathologyNo ratings yet
- Carcinogenesis Cqrcinogenic Agents 2010Document39 pagesCarcinogenesis Cqrcinogenic Agents 2010Mutiana Muspita JeliNo ratings yet
- The Genetic Basis of CancerDocument31 pagesThe Genetic Basis of Cancerapi-418176886No ratings yet
- NeoplasiaDocument36 pagesNeoplasiaaasiyaNo ratings yet
- Supplementary Materials 6.1 Cellular Aberrations-2Document11 pagesSupplementary Materials 6.1 Cellular Aberrations-2Andrea Love PalomoNo ratings yet
- Genetics, Reproduction & Female HealthcareDocument30 pagesGenetics, Reproduction & Female Healthcareronwest1990No ratings yet
- Enabling Invasion and MetastasisDocument3 pagesEnabling Invasion and MetastasisAsish GeiorgeNo ratings yet
- Ch19 Cancer Genetics1Document74 pagesCh19 Cancer Genetics1Mai Mohamed Hasan100% (1)
- Chapter 7 - NeoplasiaDocument23 pagesChapter 7 - NeoplasiaAgnieszka WisniewskaNo ratings yet
- Carcinogenesis: Robbins Basic Pathology, 7 Kumar, Cotran, RobbinsDocument29 pagesCarcinogenesis: Robbins Basic Pathology, 7 Kumar, Cotran, RobbinsHairon DhiyaulhaqNo ratings yet
- Tumors of Melanocytes Benign: (Mole) Acquired Congenital MalignantDocument73 pagesTumors of Melanocytes Benign: (Mole) Acquired Congenital Malignantlovelyc95No ratings yet
- Malignant TumorsDocument121 pagesMalignant TumorsDinesh YadavNo ratings yet
- Neoplasia Draft 2Document30 pagesNeoplasia Draft 2MuneebNo ratings yet
- CancerDocument24 pagesCancerjainsaketNo ratings yet
- ANTII CANCER DRUGS LectureDocument66 pagesANTII CANCER DRUGS Lecturebakhtawar shaikhNo ratings yet
- Cancer Biology, a Study of Cancer Pathogenesis: How to Prevent Cancer and DiseasesFrom EverandCancer Biology, a Study of Cancer Pathogenesis: How to Prevent Cancer and DiseasesNo ratings yet
- Principles Cancer Systemic TherapyDocument57 pagesPrinciples Cancer Systemic TherapyKarimina50% (2)
- Podopl in Breast CA Mod Pathol2012Document15 pagesPodopl in Breast CA Mod Pathol2012Dima PathNo ratings yet
- I Jos 201129Document9 pagesI Jos 201129jklhjNo ratings yet
- 64 - Skin TumorsDocument20 pages64 - Skin TumorsAlexa GabrielaNo ratings yet
- BreastCancer PathophysiologyDocument1 pageBreastCancer PathophysiologyClaudineNo ratings yet
- Review Imaging Appearance of Sarcomas of The ProstateDocument10 pagesReview Imaging Appearance of Sarcomas of The ProstateraannttiiNo ratings yet
- Cellular Aberrations (Basic Concepts of Oncology)Document12 pagesCellular Aberrations (Basic Concepts of Oncology)PATRIZJA YSABEL REYESNo ratings yet
- Gastro Intestinal TractDocument74 pagesGastro Intestinal Tractaeterna victrixNo ratings yet
- Ovarian TumoursDocument41 pagesOvarian TumoursVlad RazvanNo ratings yet
- The Immunobiology of Cancer: An Update ReviewDocument20 pagesThe Immunobiology of Cancer: An Update ReviewicaeeeNo ratings yet
- 02 STR 9 102Document94 pages02 STR 9 10211111No ratings yet
- السرطان هو فطر ثورة في علاج الأورام دكتور T سيمونسيني Document280 pagesالسرطان هو فطر ثورة في علاج الأورام دكتور T سيمونسيني Houda HoulaNo ratings yet
- $RUEZRZJDocument10 pages$RUEZRZJJc GaldosNo ratings yet
- Gastrointestinal MalignanciesDocument478 pagesGastrointestinal MalignanciesdrAlbertoVVNo ratings yet
- Radiotherapy: IPEM Grade A' Training PortfolioDocument9 pagesRadiotherapy: IPEM Grade A' Training PortfoliorodricesarNo ratings yet
- 17/07 TARDE (Horário 17:30 Às 18:30 H) : Cod - Res Titulo Autor1Document2 pages17/07 TARDE (Horário 17:30 Às 18:30 H) : Cod - Res Titulo Autor1Clint AnthonyNo ratings yet
- Recommendations For Cross-Sectional Imaging in Cancer Management, Second EditionDocument7 pagesRecommendations For Cross-Sectional Imaging in Cancer Management, Second EditionNedeljko TrkuljaNo ratings yet
- 360 Indonesia Fact SheetDocument2 pages360 Indonesia Fact SheetMuhammad ilyas ApotekerNo ratings yet
- Case Study 92 Breast CancerDocument3 pagesCase Study 92 Breast CancerJulia HermoginoNo ratings yet
- Principles of Radiation & Systemic Therapy-29Jan19Document17 pagesPrinciples of Radiation & Systemic Therapy-29Jan19ponderwoodNo ratings yet
- Case Study - ElectronsDocument38 pagesCase Study - Electronsapi-345373364No ratings yet
- Protocol Germ CellDocument82 pagesProtocol Germ CellTanh NguyenNo ratings yet
- Lange Qa Mammography Examination 5Th Edition Olive Peart Full ChapterDocument47 pagesLange Qa Mammography Examination 5Th Edition Olive Peart Full Chapterjennifer.miller203100% (7)
- 04 Clinical Assessment and Differential Diagnosis of A ChildDocument31 pages04 Clinical Assessment and Differential Diagnosis of A ChildAmit KavimandanNo ratings yet
- Handout - Urine and Kidney CytopathologyDocument11 pagesHandout - Urine and Kidney Cytopathologylavisha.s.punjabiNo ratings yet
- Bevacizumab in Combination With Chemotherapy For The Treatment of Advanced Ovarian CancerDocument13 pagesBevacizumab in Combination With Chemotherapy For The Treatment of Advanced Ovarian CancerPaulo Cesar Castañeda RuizNo ratings yet
- Expression of RAD6, DDB2 and Cancer Stem Cell _ CSC Protein (CD44 _sup_+__sup__CD24 _sup_-__sup_) on Therapy Response to the Administration of Chemotherapy in Ovarian Cancer_ A Prospective Flow Cytometry Study by Unedo H.M. Sihombing, Andrijono , GatoDocument11 pagesExpression of RAD6, DDB2 and Cancer Stem Cell _ CSC Protein (CD44 _sup_+__sup__CD24 _sup_-__sup_) on Therapy Response to the Administration of Chemotherapy in Ovarian Cancer_ A Prospective Flow Cytometry Study by Unedo H.M. Sihombing, Andrijono , GatoKristabella GianinaNo ratings yet
- Neproblastoma - Tumor Jinak Dan Ganas Pada Traktus Urologi 2-DITDocument9 pagesNeproblastoma - Tumor Jinak Dan Ganas Pada Traktus Urologi 2-DITNingrum JayantiNo ratings yet
- Medicineand Medical Research. 1 (1) : 24-24.: Daftar PustakaDocument6 pagesMedicineand Medical Research. 1 (1) : 24-24.: Daftar Pustakaanon_31273229No ratings yet