Professional Documents
Culture Documents
Using Resources: 4.10.4.1 Annotate The Diagram of The Haber Process Below
Using Resources: 4.10.4.1 Annotate The Diagram of The Haber Process Below
Uploaded by
Zhering RodulfoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Using Resources: 4.10.4.1 Annotate The Diagram of The Haber Process Below
Using Resources: 4.10.4.1 Annotate The Diagram of The Haber Process Below
Uploaded by
Zhering RodulfoCopyright:
Available Formats
Haber Process Question
Specification Link:
Using Resources: 4.10.4.1
Annotate the diagram of the Haber process below:
Explain why iron is used in the reactor for
the Haber process:
____________________________________
____________________________________
____________________________________
Describe how the ammonia is separated
from the other gases.:
____________________________________
____________________________________
____________________________________
What happens to the mixture of
unreacted gases (nitrogen and
hydrogen)?
__________________________________
__________________________________
__________________________________
Complete and balance the chemical
equation for the production of
ammonia from nitrogen and hydrogen.
N2 + 3 H2 __________
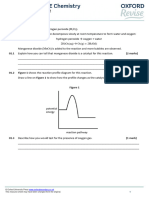
Explain what compromise conditions are The figure below shows how the equilibrium yield of
and what conditions are used in the ammonia changes with pressure at different
Haber process: temperatures.
__________________________________
__________________________________
__________________________________
__________________________________
__________________________________
__________________________________
Use the equation and your knowledge of
reversible reactions to explain why these
conditions are used in the Haber process.
.
__________________________________
__________________________________
__________________________________
__________________________________
__________________________________
__________________________________
__________________________________
__________________________________
You might also like
- BOW MATATAG Science 7 Quarter 4Document3 pagesBOW MATATAG Science 7 Quarter 4Zhering Rodulfo100% (1)
- AQA GCSE Triple C6 Test 6 Advanced QPDocument22 pagesAQA GCSE Triple C6 Test 6 Advanced QPryanNo ratings yet
- Rigid and Flexible Pavemment FormulasDocument13 pagesRigid and Flexible Pavemment FormulasRonald Costales100% (2)
- Soalan Sains f1Document10 pagesSoalan Sains f1Armadaramor ShiNo ratings yet
- AQA GCSE Triple C10 Test 6 Advanced QPDocument14 pagesAQA GCSE Triple C10 Test 6 Advanced QPryanNo ratings yet
- Alcohol Homologous SeriesDocument5 pagesAlcohol Homologous SeriesChia Sann Roo CynthiaNo ratings yet
- Language of Chemistry - Worksheet 4Document2 pagesLanguage of Chemistry - Worksheet 4movies gamesNo ratings yet
- Paper 2 (Pract Paper)Document19 pagesPaper 2 (Pract Paper)ephixNo ratings yet
- C11 Worksheet IDocument6 pagesC11 Worksheet Inguyenlucas1301No ratings yet
- CSEC Type Exam Style Questions 2 Paper 2Document8 pagesCSEC Type Exam Style Questions 2 Paper 2da3327017No ratings yet
- Carbonyls Ppqs NO ANSWERSDocument9 pagesCarbonyls Ppqs NO ANSWERSmariam saikNo ratings yet
- Acids and Bases: Section 4 NeutralizationDocument4 pagesAcids and Bases: Section 4 NeutralizationDevine RawlsNo ratings yet
- BOT 20 Laboratory Exercise 1Document1 pageBOT 20 Laboratory Exercise 1Lukas BlancoNo ratings yet
- Igcse Chemistry 5ed TR End of Chapter Test 9Document3 pagesIgcse Chemistry 5ed TR End of Chapter Test 9Marin PesicNo ratings yet
- VIII-Chemistry-Revision PWS-2-Unit 11 and 13Document4 pagesVIII-Chemistry-Revision PWS-2-Unit 11 and 13anousha1488No ratings yet
- Fractional Distillation QDocument9 pagesFractional Distillation Qarychan418No ratings yet
- F5S Chemistry Revision Worksheet (6) - Double DecompositionDocument8 pagesF5S Chemistry Revision Worksheet (6) - Double DecompositionRaymond ChanNo ratings yet
- AQA GCSE Triple C6 Test 5 Advanced QPDocument18 pagesAQA GCSE Triple C6 Test 5 Advanced QPryanNo ratings yet
- Alcohols PackDocument15 pagesAlcohols PackbilaalquadriNo ratings yet
- Cracking Hydrocarbons Practice QuestionDocument1 pageCracking Hydrocarbons Practice QuestionZhering RodulfoNo ratings yet
- Organics 1 Triple 2Document46 pagesOrganics 1 Triple 2AnonymousNo ratings yet
- CHEMISTRY PAPER 1 (Inorganic & Physical) : University Predictor Examination 2018Document13 pagesCHEMISTRY PAPER 1 (Inorganic & Physical) : University Predictor Examination 2018Lisa PintoNo ratings yet
- Sep 2013Document28 pagesSep 2013Dylan EllulNo ratings yet
- Cracking AnswersDocument6 pagesCracking AnswersPaul BurgessNo ratings yet
- Crop 2030Document12 pagesCrop 2030Nqobile SingojwanaNo ratings yet
- UntitledDocument16 pagesUntitledMichel ElizeeNo ratings yet
- 2022 Mock CHM 0715 P2 FinalDocument11 pages2022 Mock CHM 0715 P2 FinalblessingyakumNo ratings yet
- IGCSE Chemistry Section 5 SeparateDocument10 pagesIGCSE Chemistry Section 5 SeparateSoraya DeenNo ratings yet
- Rates & Atmos QPDocument34 pagesRates & Atmos QPlizablatchfordNo ratings yet
- Activity No. 1.2 Determination of The Chemical Formula of A HydrateDocument4 pagesActivity No. 1.2 Determination of The Chemical Formula of A HydrateOptional AlternateNo ratings yet
- Third Sequence Lwer SixthDocument4 pagesThird Sequence Lwer SixthNgah Lilwaine MNo ratings yet
- Revision Sheet POM ANSWERSDocument7 pagesRevision Sheet POM ANSWERSMeena KulkarniNo ratings yet
- Organic Chemistry: WorksheetDocument50 pagesOrganic Chemistry: Worksheetshahed khayyatNo ratings yet
- Experiment 1.1 Data SheetDocument2 pagesExperiment 1.1 Data Sheetdharleene fionaNo ratings yet
- QuestionsDocument1 pageQuestionsAlan Paul Dela CruzNo ratings yet
- Paper 2 Past Paper Pack Y13 2023Document51 pagesPaper 2 Past Paper Pack Y13 2023Xx Jasmine XxNo ratings yet
- Science Coursebook QuestionsDocument11 pagesScience Coursebook QuestionsG10-02-16-IM SIHYUNNo ratings yet
- CC1 2 Revision MatDocument2 pagesCC1 2 Revision MatMysticalNo ratings yet
- WS2 IG I Chemistry (1) SEPERATING MIXTURESDocument4 pagesWS2 IG I Chemistry (1) SEPERATING MIXTURESRaj MalkanNo ratings yet
- Act03 Membrane Transport ExerciseDocument4 pagesAct03 Membrane Transport ExerciseBea ENo ratings yet
- Std. X Chemistry PAPER IV ExtendedDocument8 pagesStd. X Chemistry PAPER IV ExtendedYashodhaNo ratings yet
- IB Chem Practice WorksheetsDocument7 pagesIB Chem Practice WorksheetsSamira NamavarNo ratings yet
- Trial PT3 Sains 2015 (1) Simpang BekohDocument18 pagesTrial PT3 Sains 2015 (1) Simpang BekohHalizah RamthanNo ratings yet
- Grade 9 Chem First Term ExamDocument7 pagesGrade 9 Chem First Term ExamLarry MofaNo ratings yet
- Catalysts PhysicalchemistryDocument45 pagesCatalysts Physicalchemistry/ “Nu” /No ratings yet
- 3.3.9.2 Acylation (A-Level Only)Document95 pages3.3.9.2 Acylation (A-Level Only)jason smtihNo ratings yet
- Science July Assignment Grade 8Document3 pagesScience July Assignment Grade 8G PNo ratings yet
- Physics Y10Document2 pagesPhysics Y10vikramrolex96No ratings yet
- f.1 Chem Cycle 1 Term 3 2016Document10 pagesf.1 Chem Cycle 1 Term 3 2016Vincent AgumbaNo ratings yet
- L3 Exam Questions 7-12-21Document12 pagesL3 Exam Questions 7-12-21boobooNo ratings yet
- Ammonia: Questions: Figure: The Equilibrium Conversion To Ammonia Under Different ConditionsDocument3 pagesAmmonia: Questions: Figure: The Equilibrium Conversion To Ammonia Under Different ConditionsJai-Michael FrancisNo ratings yet
- AQA GCSE Triple C8 Test 6 Advanced QPDocument13 pagesAQA GCSE Triple C8 Test 6 Advanced QPryanNo ratings yet
- Chemistry Upper Sixth 2016 First SequenceDocument7 pagesChemistry Upper Sixth 2016 First SequenceNgah Lilwaine MNo ratings yet
- c4 Titrations Chem OnlyDocument31 pagesc4 Titrations Chem OnlyMadhavi OchaniNo ratings yet
- Thermal IIIqsDocument14 pagesThermal IIIqsjingcong liuNo ratings yet
- Intro To Atoms Moles and Stoichiometry: As Level Chemistry Test Name: Class: TeacherDocument8 pagesIntro To Atoms Moles and Stoichiometry: As Level Chemistry Test Name: Class: TeacherMatthew James PopeNo ratings yet
- NewDocument1 4Document30 pagesNewDocument1 4Rishikesh MaharajNo ratings yet
- PBD Exercise Chapter 6Document2 pagesPBD Exercise Chapter 6syuhada zakariaNo ratings yet
- GFE PPQs StudentDocument7 pagesGFE PPQs StudentRahbot Wolde-MichaelNo ratings yet
- Nzic 2017 2.6Document7 pagesNzic 2017 2.6helensh008No ratings yet
- Hacking for Beginners: Comprehensive Guide on Hacking Websites, Smartphones, Wireless Networks, Conducting Social Engineering, Performing a Penetration Test, and Securing Your Network (2022)From EverandHacking for Beginners: Comprehensive Guide on Hacking Websites, Smartphones, Wireless Networks, Conducting Social Engineering, Performing a Penetration Test, and Securing Your Network (2022)No ratings yet
- College Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsFrom EverandCollege Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsNo ratings yet
- Respiratory SystemDocument4 pagesRespiratory SystemZhering RodulfoNo ratings yet
- TLE - Technical Drafting (Region X)Document38 pagesTLE - Technical Drafting (Region X)Zhering RodulfoNo ratings yet
- ScienceDocument27 pagesScienceZhering RodulfoNo ratings yet
- Acid-With-Metals-Worksheet ZDocument1 pageAcid-With-Metals-Worksheet ZZhering RodulfoNo ratings yet
- Science Earth Science 11 Q1Document10 pagesScience Earth Science 11 Q1Zhering RodulfoNo ratings yet
- LR DLP Circulatory SystemDocument21 pagesLR DLP Circulatory SystemZhering RodulfoNo ratings yet
- Carbohydrate Practice QuestionDocument1 pageCarbohydrate Practice QuestionZhering RodulfoNo ratings yet
- PE and Health 10 Q1Document13 pagesPE and Health 10 Q1Zhering RodulfoNo ratings yet
- Heat Transfer COT g7Document42 pagesHeat Transfer COT g7Zhering RodulfoNo ratings yet
- Science Gen Physics 12 Q1Document14 pagesScience Gen Physics 12 Q1Zhering RodulfoNo ratings yet
- Science Biology 11-12 Q1Document9 pagesScience Biology 11-12 Q1Zhering RodulfoNo ratings yet
- Tech Science LiteracyDocument36 pagesTech Science LiteracyZhering RodulfoNo ratings yet
- Applications of Heat TransferDocument7 pagesApplications of Heat TransferZhering RodulfoNo ratings yet
- 3rd Quarter PHYSICAL SCIENCE ExamDocument19 pages3rd Quarter PHYSICAL SCIENCE ExamZhering RodulfoNo ratings yet
- Communicable Diseases: 4.1.1: Explain The Importance in Terms On Long Term Immunity of Memory B CellsDocument1 pageCommunicable Diseases: 4.1.1: Explain The Importance in Terms On Long Term Immunity of Memory B CellsZhering RodulfoNo ratings yet
- Enzymes Practice QuestionDocument1 pageEnzymes Practice QuestionZhering RodulfoNo ratings yet
- Checklist: Before You Start Have You Got The Following: 5/4.4.3 ElectrolysisDocument2 pagesChecklist: Before You Start Have You Got The Following: 5/4.4.3 ElectrolysisZhering Rodulfo100% (1)
- Cracking Hydrocarbons Practice QuestionDocument1 pageCracking Hydrocarbons Practice QuestionZhering RodulfoNo ratings yet
- Ionic Bonding Practice QuestionDocument1 pageIonic Bonding Practice QuestionZhering RodulfoNo ratings yet
- Chemical Changes Game Design InstructionsDocument2 pagesChemical Changes Game Design InstructionsZhering RodulfoNo ratings yet
- Antibodies Practice QuestionDocument1 pageAntibodies Practice QuestionZhering RodulfoNo ratings yet
- En - mb997 F407VGT6 E01 SchematicDocument9 pagesEn - mb997 F407VGT6 E01 SchematicBharath Kumar Reddy MNo ratings yet
- 11.7 States of Matter PhET LabDocument2 pages11.7 States of Matter PhET LabCoacytTucumanNo ratings yet
- Oando For Jevlink2Document4 pagesOando For Jevlink2Steve Bassey100% (2)
- PA - StocksDocument36 pagesPA - Stocksikolev57No ratings yet
- Blue Pelican Java Textbook by Charles E. CookDocument543 pagesBlue Pelican Java Textbook by Charles E. CookAlex PopaNo ratings yet
- DLP IN ORAL COMMUNICATION-GRADE 11-EditedDocument6 pagesDLP IN ORAL COMMUNICATION-GRADE 11-EditedMaeann MirandoNo ratings yet
- Imam Ali High School/Mr El Bahi/As-Lessons/Relative Clauses Relative ClausesDocument2 pagesImam Ali High School/Mr El Bahi/As-Lessons/Relative Clauses Relative ClausesEnglish for everyoneNo ratings yet
- XZXDocument2 pagesXZXImran Sajid ShahidNo ratings yet
- Cap1 - Engineering in TimeDocument12 pagesCap1 - Engineering in TimeHair Lopez100% (1)
- BankingDocument99 pagesBankingNamrata KulkarniNo ratings yet
- TSQL Coding Standards ChecklistDocument5 pagesTSQL Coding Standards ChecklistSaman AzeemNo ratings yet
- Krishna PDFCVDocument4 pagesKrishna PDFCVAVS InfraNo ratings yet
- 1700 Kva Rab Panel SinkronDocument2 pages1700 Kva Rab Panel SinkronIqbal KomengNo ratings yet
- Micro paraDocument7 pagesMicro paraAj MillanNo ratings yet
- Plugin For Sketchup Cleanup - Download Tutorial Sketchup (PDFDrive)Document46 pagesPlugin For Sketchup Cleanup - Download Tutorial Sketchup (PDFDrive)sham_codeNo ratings yet
- Tally Assignment 12Document90 pagesTally Assignment 12Kaushal SharmaNo ratings yet
- Induction Machine ModellingDocument23 pagesInduction Machine ModellingKishan Darji100% (1)
- DS 113 Science Technology and Innovation For DevelopmentDocument50 pagesDS 113 Science Technology and Innovation For DevelopmentEldard KafulaNo ratings yet
- Astrovega May 2024Document44 pagesAstrovega May 2024VARA PRASADNo ratings yet
- Unit 10 Shape and Space Objective Questions 1: GHI Are Equal in LengthDocument12 pagesUnit 10 Shape and Space Objective Questions 1: GHI Are Equal in LengthhairoldinNo ratings yet
- APICS BSCM Chapter 1 NotesDocument11 pagesAPICS BSCM Chapter 1 NotesvayugaramNo ratings yet
- By: Saurabh S SawhneyDocument28 pagesBy: Saurabh S SawhneyMaria100% (1)
- Access Grammar 2Document20 pagesAccess Grammar 2Agostina Calienno PazNo ratings yet
- Let's Get Scotland Walking - The National Walking Strategy - Escócia - 2019Document29 pagesLet's Get Scotland Walking - The National Walking Strategy - Escócia - 2019Natália CostaNo ratings yet
- CH-5 Laws of MotionDocument23 pagesCH-5 Laws of MotionAkash GuptaNo ratings yet
- Stage 1 English Curriculum Framework PDFDocument2 pagesStage 1 English Curriculum Framework PDFMangunatun KhasanahNo ratings yet
- HVAC and Refrigeration SystemDocument36 pagesHVAC and Refrigeration Systemranveer100% (1)
- Problem Solving ActivitiesDocument6 pagesProblem Solving ActivitiesreginafernNo ratings yet
- Diabetes Mellitus Diagnosis Using Optical Ring Resonators-758Document7 pagesDiabetes Mellitus Diagnosis Using Optical Ring Resonators-758Darshan rajNo ratings yet