Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
38 viewsTemperature and Heat: Powerpoint Lectures For
Temperature and Heat: Powerpoint Lectures For
Uploaded by
Mark Ronald SuaisoCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You might also like
- Physics IA For The IB 2019Document9 pagesPhysics IA For The IB 2019Aarav verma75% (12)
- Physical Science (Specialization) Reviewer 494 Items With RationalizationDocument118 pagesPhysical Science (Specialization) Reviewer 494 Items With RationalizationHazel Climacosa Tuprio100% (4)
- Genesis CSEC Physics 2010-2018 SolutionsDocument110 pagesGenesis CSEC Physics 2010-2018 SolutionsBill Bob80% (5)
- Ch. 20 - Second Law of ThermodynamicsDocument36 pagesCh. 20 - Second Law of ThermodynamicsMark Ronald SuaisoNo ratings yet
- HAZIDDocument19 pagesHAZIDRogelio Lazo ArjonaNo ratings yet
- Topic 3.1 FormativeDocument2 pagesTopic 3.1 FormativeDharmesh Ramnarayan YadavNo ratings yet
- Assignment 1 & 2Document17 pagesAssignment 1 & 2Iyyan Paramanandam67% (3)
- Temperature and Heat: Powerpoint Lectures ForDocument32 pagesTemperature and Heat: Powerpoint Lectures ForAbdullah ZafarNo ratings yet
- 6-New Thermal Handout Csec 2021Document18 pages6-New Thermal Handout Csec 2021Nirvan RampersadNo ratings yet
- Temperature-WPS OfficeDocument9 pagesTemperature-WPS OfficeVhenz MapiliNo ratings yet
- Thermodynamics Module 5: Assessment Task 5: Laguna University College of Education A.Y 2020-2021Document20 pagesThermodynamics Module 5: Assessment Task 5: Laguna University College of Education A.Y 2020-2021anembam putobungbongNo ratings yet
- Heat CompleteDocument24 pagesHeat CompletePhilip MooreNo ratings yet
- Science10 Q4week1-2Document32 pagesScience10 Q4week1-2ClyzuhNo ratings yet
- Heat and ThermodynamicsDocument45 pagesHeat and ThermodynamicsbairojushivakumarNo ratings yet
- ThermodynamicsDocument78 pagesThermodynamicsHarold Jan R. TeranoNo ratings yet
- Thermal EnergyDocument29 pagesThermal EnergygerryamamooNo ratings yet
- Heat 2Document20 pagesHeat 2Sydney LezamaNo ratings yet
- QuestionsDocument2 pagesQuestionsMEOW41No ratings yet
- Temperature, Thermometry, Thermal Expansion & Gas LawsDocument50 pagesTemperature, Thermometry, Thermal Expansion & Gas LawsEmmanuel AdeyemiNo ratings yet
- Thermal ExpansionDocument18 pagesThermal ExpansionElleNo ratings yet
- Experiment 302 Heat and CalorimetryDocument2 pagesExperiment 302 Heat and CalorimetryJasmin DionisioNo ratings yet
- Chapter 10Document44 pagesChapter 10Jayaletchumi T. Sambantha MoorthyNo ratings yet
- 4Q W1 Kinetic Molecular Theory of Gases, Boyle's and Charles' LawsDocument54 pages4Q W1 Kinetic Molecular Theory of Gases, Boyle's and Charles' Lawsjia aganaNo ratings yet
- TDA301T Lesson 4 Equations of StateDocument60 pagesTDA301T Lesson 4 Equations of StateBrown Zuzu LesapoNo ratings yet
- Bses 29 ReviewerDocument8 pagesBses 29 Reviewerjerico.lapurgaNo ratings yet
- 11 ChapterDocument24 pages11 ChapterShashwat SahayNo ratings yet
- Warming The Earth and The AtmosphereDocument46 pagesWarming The Earth and The AtmosphereKylene AlimNo ratings yet
- Basic Heat 103Document48 pagesBasic Heat 103jesseamobi10No ratings yet
- Chapter 10 Thermal Properties of MatterDocument39 pagesChapter 10 Thermal Properties of MatterAnil KumarNo ratings yet
- Physics 2 1Document104 pagesPhysics 2 1Kimberly GonzalesNo ratings yet
- Changes Due To TemperatureDocument2 pagesChanges Due To TemperaturePradnyaParamitaDewiMrzNo ratings yet
- LO10 Session 08 ThermodynamicsDocument44 pagesLO10 Session 08 ThermodynamicsAbo Alphotoh GamingNo ratings yet
- Applied Physics: Lec - 1: Introduction To ThermodynamicsDocument37 pagesApplied Physics: Lec - 1: Introduction To ThermodynamicsTayyaba TanveerNo ratings yet
- Transformation by SteamDocument43 pagesTransformation by SteamLlama jennerNo ratings yet
- P103 Chapter10 TAT wk3wk4Document62 pagesP103 Chapter10 TAT wk3wk4Muhammad SaeedNo ratings yet
- Thermal Expansion of Solid Bodies by Youssef FINIANOSDocument15 pagesThermal Expansion of Solid Bodies by Youssef FINIANOSYoussefNo ratings yet
- Si UnitsDocument92 pagesSi UnitsRAVI2296No ratings yet
- Thermal Properties of Matter: Group 5Document40 pagesThermal Properties of Matter: Group 5LIYA ASKARNo ratings yet
- Website For This Class: Homework Will Be Posted at The: Phys5DDocument23 pagesWebsite For This Class: Homework Will Be Posted at The: Phys5DReeja MathewNo ratings yet
- Basic Refrigeration System - MATTERDocument10 pagesBasic Refrigeration System - MATTERCisco StarkNo ratings yet
- Physics ProjectDocument19 pagesPhysics Projectaditya varteNo ratings yet
- 1.Dr - Ahmed Samy - PhysicsDocument21 pages1.Dr - Ahmed Samy - PhysicsKhaled AhmedNo ratings yet
- C1-States of MatterDocument116 pagesC1-States of MatterGivemore MuromboNo ratings yet
- Temperature and Heat ComplteDocument62 pagesTemperature and Heat ComplteSophia BandojaNo ratings yet
- Materi Fisika AdipaDocument4 pagesMateri Fisika AdipaI Putu Adi Payana PutraNo ratings yet
- Module Thermodynamics 1-4: Colorado State UniversityDocument28 pagesModule Thermodynamics 1-4: Colorado State UniversityAsyifa Rizqi UtamiNo ratings yet
- Special Cases of The Ideal Gas LawDocument8 pagesSpecial Cases of The Ideal Gas LawJohn Benedick Beri BernalNo ratings yet
- LectureFME16Materials Science and Engineering4Document14 pagesLectureFME16Materials Science and Engineering4Cllyan ReyesNo ratings yet
- Heat and TemperatureDocument6 pagesHeat and TemperatureJavontay StewartNo ratings yet
- PHY12L E302Document4 pagesPHY12L E302Mikaella TambisNo ratings yet
- Warming The Earth and The AtmosphereDocument46 pagesWarming The Earth and The AtmosphereSalih TrexieNo ratings yet
- Temperature SensorsDocument181 pagesTemperature Sensorsbhaihello015No ratings yet
- CSEC Physics 12 Hour Crash Course Sections B and C Student Version-2Document107 pagesCSEC Physics 12 Hour Crash Course Sections B and C Student Version-2Mrs MendezNo ratings yet
- Temperature Heat Transfer: AbsorptionDocument4 pagesTemperature Heat Transfer: AbsorptionFredalyn Joy VelaqueNo ratings yet
- 4 Thermal Properties and TemperatureDocument32 pages4 Thermal Properties and Temperatureamgedalaa118No ratings yet
- Nature of Heat & Temperature 2022Document22 pagesNature of Heat & Temperature 2022Gabriella SteeleNo ratings yet
- Heat and TemperatureDocument52 pagesHeat and TemperatureAngilyn LumabasNo ratings yet
- Chapter Ten Lecture Ten Thermodynamics: TemperatureDocument16 pagesChapter Ten Lecture Ten Thermodynamics: TemperatureTony AtefNo ratings yet
- Science Script: Scene 1Document4 pagesScience Script: Scene 1Mikaela MercadoNo ratings yet
- NCERT PH 2 Thermal Properties of MatterDocument24 pagesNCERT PH 2 Thermal Properties of MatterkdsiddhantNo ratings yet
- Thermal PhysicsDocument51 pagesThermal PhysicsJerrySemuel100% (2)
- Thermodynamic Principles: Topic 1Document32 pagesThermodynamic Principles: Topic 1Jinang ShahNo ratings yet
- LF Thermal ExpansionDocument31 pagesLF Thermal ExpansionLalaine RamosNo ratings yet
- Chapter 4:physics:form 4Document10 pagesChapter 4:physics:form 4jiivi87No ratings yet
- Che 110 Exp 14Document8 pagesChe 110 Exp 14virgobabii16No ratings yet
- Temperature, Thermometry, Thermal Expansion & Gas LawsDocument52 pagesTemperature, Thermometry, Thermal Expansion & Gas LawsOlamide AyindeNo ratings yet
- Sheet - A 21 75 - Helipad Stairs DetailDocument1 pageSheet - A 21 75 - Helipad Stairs DetailMark Ronald SuaisoNo ratings yet
- Portfolio Value at Risk Commission Stock Cut Loss % 25000 250 1.19% 2000Document2 pagesPortfolio Value at Risk Commission Stock Cut Loss % 25000 250 1.19% 2000Mark Ronald SuaisoNo ratings yet
- Ch. 23 - Electric PotentialDocument93 pagesCh. 23 - Electric PotentialMark Ronald SuaisoNo ratings yet
- Gauss'S Law: Powerpoint Lectures ForDocument84 pagesGauss'S Law: Powerpoint Lectures ForMark Ronald SuaisoNo ratings yet
- Ch. 18 - Thermal Properties of MatterDocument72 pagesCh. 18 - Thermal Properties of MatterMark Ronald SuaisoNo ratings yet
- Mechanics - Theory: Variable Location For Multiple Point LoadsDocument5 pagesMechanics - Theory: Variable Location For Multiple Point LoadsMark Ronald SuaisoNo ratings yet
- Ch. 7 - Potential Energy and Energy ConservationsDocument25 pagesCh. 7 - Potential Energy and Energy ConservationsMark Ronald SuaisoNo ratings yet
- Sine and Cosine Law: Name: - DateDocument2 pagesSine and Cosine Law: Name: - DateMark Ronald SuaisoNo ratings yet
- Ch. 9 - Rotation of Rigid BodiesDocument23 pagesCh. 9 - Rotation of Rigid BodiesMark Ronald SuaisoNo ratings yet
- Ch. 8 Momentum, Impulse and CollsionsDocument29 pagesCh. 8 Momentum, Impulse and CollsionsMark Ronald SuaisoNo ratings yet
- Pig Breeding CalculatorDocument14 pagesPig Breeding CalculatorMark Ronald SuaisoNo ratings yet
- TutorMe ApplicationDocument2 pagesTutorMe ApplicationMark Ronald SuaisoNo ratings yet
- Ch. 5 - Applying Newtons LawsDocument21 pagesCh. 5 - Applying Newtons LawsMark Ronald SuaisoNo ratings yet
- Ch. 6 - Work and Kinetic EnergyDocument20 pagesCh. 6 - Work and Kinetic EnergyMark Ronald SuaisoNo ratings yet
- Ch. 3 - Motion in Two or Three DimensionsDocument33 pagesCh. 3 - Motion in Two or Three DimensionsMark Ronald SuaisoNo ratings yet
- Penrose Platter Spin Ices - Artificial Magnetic QuasicrystalsDocument38 pagesPenrose Platter Spin Ices - Artificial Magnetic QuasicrystalsEvelyn JonesNo ratings yet
- Thermotherapy PDFDocument51 pagesThermotherapy PDFRUdraNo ratings yet
- Thermodynamics Part 1 by Shobhit NirwanDocument14 pagesThermodynamics Part 1 by Shobhit NirwanKumar Okumura90% (10)
- Experiment No. 2 - Specific Heat (For 2024 - IIA APDEV)Document6 pagesExperiment No. 2 - Specific Heat (For 2024 - IIA APDEV)ladyarboleda26No ratings yet
- Adiabatic FBR DesignDocument10 pagesAdiabatic FBR DesignRana UzairNo ratings yet
- Chapter # 11 Heat & Thermodynamic Golden PointsDocument8 pagesChapter # 11 Heat & Thermodynamic Golden PointsMudasir Hussain TuriNo ratings yet
- Physics Form 4 Yearly Lesson PlanDocument22 pagesPhysics Form 4 Yearly Lesson PlanArfa Suhaida ZainNo ratings yet
- ChemDocument38 pagesChemCHLOE BEA DILLANo ratings yet
- Chapter 7 Continued Entropy: A Measure of Disorder Study Guide in PowerpointDocument53 pagesChapter 7 Continued Entropy: A Measure of Disorder Study Guide in Powerpointbrayan CortezNo ratings yet
- Access and Utilization of The Dippr Physical Property Database in Ch. E. EducationDocument12 pagesAccess and Utilization of The Dippr Physical Property Database in Ch. E. Educationingbarragan87No ratings yet
- Basic Concepts of Thermodynamics (Animation) - YouTube - EnglishDocument4 pagesBasic Concepts of Thermodynamics (Animation) - YouTube - EnglishtitojhezielanneNo ratings yet
- Energetics QuestionsDocument58 pagesEnergetics QuestionsQasim Peracha100% (1)
- MM231 LecturesDocument254 pagesMM231 LecturesMansour AbbasNo ratings yet
- Fiberfrax Blanket and Mat Products: Product Information SheetDocument8 pagesFiberfrax Blanket and Mat Products: Product Information SheetMagin Idelfonso TorreblancaNo ratings yet
- JR AIIMS S60 - NEET WET - 21 (22-11-21) SYLLABUSDocument1 pageJR AIIMS S60 - NEET WET - 21 (22-11-21) SYLLABUSCR RaghavNo ratings yet
- I.C EngineDocument154 pagesI.C EngineShafiq AhmadNo ratings yet
- Experiment 6 - Specific Heat CapacityDocument27 pagesExperiment 6 - Specific Heat CapacitydaNo ratings yet
- Topic 5 HeatDocument26 pagesTopic 5 HeatAnthonyDomNo ratings yet
- Chapter 2Document29 pagesChapter 2Bilal shahzadNo ratings yet
- 152 TOP Thermodynamics - Mechanical Engineering Multiple Choice Questions and Answers ListDocument30 pages152 TOP Thermodynamics - Mechanical Engineering Multiple Choice Questions and Answers ListSampat AgnihotriNo ratings yet
- Concise Guide To Heat Exchanger Network Design: Xian Wen NGDocument154 pagesConcise Guide To Heat Exchanger Network Design: Xian Wen NGFIRSON ANDRES SerranoNo ratings yet
- CHEN 200 Homework Problems 1: Problem 1: (3 Marks)Document2 pagesCHEN 200 Homework Problems 1: Problem 1: (3 Marks)Amy MillerNo ratings yet
- Introduction To Chemical Engineering Thermodynamics J M Smith Full ChapterDocument51 pagesIntroduction To Chemical Engineering Thermodynamics J M Smith Full Chapteragnes.baer637100% (8)
Temperature and Heat: Powerpoint Lectures For
Temperature and Heat: Powerpoint Lectures For
Uploaded by
Mark Ronald Suaiso0 ratings0% found this document useful (0 votes)
38 views26 pagesOriginal Title
Ch. 17 - Temperature and Heat
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
Download as ppt, pdf, or txt
0 ratings0% found this document useful (0 votes)
38 views26 pagesTemperature and Heat: Powerpoint Lectures For
Temperature and Heat: Powerpoint Lectures For
Uploaded by
Mark Ronald SuaisoCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
Download as ppt, pdf, or txt
You are on page 1of 26
Chapter 17
Temperature and Heat
PowerPoint® Lectures for
University Physics, Twelfth Edition
– Hugh D. Young and Roger A. Freedman
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Goals for Chapter 17
• To delineate the three different temperature scales
• To describe thermal expansion and thermal stress
• To consider heat, phase changes, and calorimetry
• To study how heat flows with convection,
conduction, and radiation
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Introduction
• Growing up in Pittsburgh, molten
steel was a common sight. Still it
is imposing at 1500oC.
• The worst common burns you can
imagine are steam burns. You
have not only water heated to its
boiling point but gaseous steam
carrying the heat of vaporization.
It’s a great deal of energy in a
small space.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Measuring temperature
• There are many ways to measure
temperature, but the two devices
mentioned below take advantage of
a gas or liquid sample which
expands if heat is added and
contracts if heat is removed.
• A cylinder of gas will show pressure
rise if volume is kept constant.
• A small container of liquid will see
the liquid increase in volume as
temperatures rise. Mercury was
chosen “early on” because it’s so
dense, a small volume can record
large temperature ranges.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
The zeroth law of thermodynamics
• Simply stated? “Heat will always travel from a hot reservoir to a cold
one without outside energy forcing an unnatural transfer.”
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Thermometers? Just our way of trying to see where the heat is
• The measure of
temperature is a way of
expressing how much
heat one object is
holding relative to
another.
• There are several
examples shown at
right. You can base a
thermometer on thermal
expansion of a gas,
differential expansion
of bimetal strips, even
on something as wild as
laser-doppler shift.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
The coldest we can ever get?
• Early experiments observed changes in pressure or volume as
temperature changed.
• It was noticed that the linear trends lead to a consistent lowest
temperature that we call “absolute zero”—labeled 0K after Lord Kelvin.
• Refer to Example 17.1.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Conversions are expected
• Values on the temperatures scales (Fahrenheit, Centigrade/Celsius,
and Kelvin) may be readily interconverted. Physics professors will
want values to eventually be in Kelvins because that’s the form in
SI units.
• See Figure 17.7 below.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
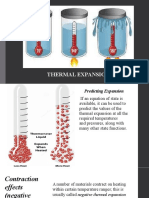
Thermal expansion—linear
• A change in
length will
accompany a
change in
temperature.
The size of the
change will
depend on the
material.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Changing temperature changes atomic spacing
• Molecules can be visualized as bedsprings and spheres. More
heat (higher temperatures) is reflected by the motion of the
atoms relative to each other.
• See Figure 17.9 below.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Coefficients of expansion
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Thermal changes in material length and volume
• Refer to Problem-Solving Strategy 17.1.
• Consult Example 17.2 (change in length).
• Consult Example 17.3 (change in length II).
• Consult Example 17.4 (change in volume).
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Thermal expansion we see constantly
• Water is interesting. There are no
other liquids that expand to become
less dense as a solid than they are
as a liquid. This is fortunate, if
lakes were to freeze and dense ice
sink to the bottom, everything in
the water would die as the liquid
became solid from the bottom up.
• Thermal expansion joints allow
roads to expand and contract
without any stress to the material
used to build.
• Refer to Example 17.5.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
James Joule and the mechanical equivalent of heat
• Joule knew a mass
above the ground had
potential energy. He
dropped an object on a
cord, turning a paddle
in water monitored by
a very accurate
thermometer.
• His conclusion was to
connect energy
conservation (potential
and kinetic) to heat as
a third form observed.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Specific heat
• A specific heat value reveals
how much temperature will
change when a given amount of
a substance absorbs a given
amount of heat.
• Water is a “benchmark” as one
ml of water will absorb 1 cal of
heat to raise its temperature by
1oC.
• Refer to Example 17.6 and
Example 17.7.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Specific heat values
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Phase changes and temperature behavior
• A solid will absorb heat according to its heat
capacity, becoming a hotter solid.
• At the melting point, a solid will absorb its
heat of fusion and become a liquid. An
equilibrium mixture of a substance in both its
liquid and solid phases will have a constant
temperature.
• A cold liquid will absorb heat according to its
heat capacity to become a hotter liquid.
• At the boiling point, a liquid will absorb its
heat of vaporization and become a gas. An
equilibrium mixture of liquid and gas will have
a constant temperature.
• A cold gas can absorb heat according to its
heat capacity and become a hotter gas.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Heats of Fusion and Heats of Vaporization
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Using well-behaved water to measure other systems
• Because water is a good thermal sink, is readily
available, and reproducibly absorbs 4.184 J for
every gram to rise in temperature by 1oC, it is
often used to measure another object’s change in
heat energy by comparison.
• For example, an unknown metal might be
massed, raised to a known temperature (say to
100oC in a boiling water bath), then added to a
known amount of cold water. The resulting
change in the temperature of the water will allow
heat absorbed to be calculated and then the heat
capacity of the unknown metal.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Heat calculations
• Follow Problem-Solving Strategy 17.2.
• Refer to Example 17.8 (no phase change).
• Refer to Example 17.9 (changes in both temperature and
phase).
• Refer to Example 17.10 (an example that could be done in a
kitchen).
• Refer to Example 17.11 (combustion, temperature change,
and phase change).
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Why, and how well, do materials transfer heat?
• Figure 17.23 illustrates
the model.
• Table 17.5 lists thermal
conductivities. They are
dramatically different,
from very large values
for conductors like
metals to very small
values for insulators
like styrofoam or wood.
• Consider Problem-
Solving Strategy 17.3.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Conduction of heat I
• Consider Example 17.12.
• What makes a picnic
cooler effective?
• Figure 17.25 at right
illustrates the problem.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Conduction of heat II
• Consider Example 17.13.
• This is a good reason not to pick up a metal frying pan by
its bare handle.
• Figure 17.26 below illustrates the problem.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Conduction of heat III
• Consider Example 17.14.
• There are variations of the metal bar problem.
• Figure 17.27 below illustrates the problem.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Convection of heat
• Heating by moving large
amounts of hot fluid,
usually water or air.
• Figure 17.28 at right
illustrates heat moving by
convection.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
Radiation of heat
• Infrared lights, hot metal
objects, a fireplace,
standing near a running
furnace … these are all
objects heating others by
broadcast of EM radiation
just lower in energy than
visible red.
• Consider Example 17.15.
• Consider Example 17.16.
Copyright © 2008 Pearson Education Inc., publishing as Pearson Addison-Wesley
You might also like
- Physics IA For The IB 2019Document9 pagesPhysics IA For The IB 2019Aarav verma75% (12)
- Physical Science (Specialization) Reviewer 494 Items With RationalizationDocument118 pagesPhysical Science (Specialization) Reviewer 494 Items With RationalizationHazel Climacosa Tuprio100% (4)
- Genesis CSEC Physics 2010-2018 SolutionsDocument110 pagesGenesis CSEC Physics 2010-2018 SolutionsBill Bob80% (5)
- Ch. 20 - Second Law of ThermodynamicsDocument36 pagesCh. 20 - Second Law of ThermodynamicsMark Ronald SuaisoNo ratings yet
- HAZIDDocument19 pagesHAZIDRogelio Lazo ArjonaNo ratings yet
- Topic 3.1 FormativeDocument2 pagesTopic 3.1 FormativeDharmesh Ramnarayan YadavNo ratings yet
- Assignment 1 & 2Document17 pagesAssignment 1 & 2Iyyan Paramanandam67% (3)
- Temperature and Heat: Powerpoint Lectures ForDocument32 pagesTemperature and Heat: Powerpoint Lectures ForAbdullah ZafarNo ratings yet
- 6-New Thermal Handout Csec 2021Document18 pages6-New Thermal Handout Csec 2021Nirvan RampersadNo ratings yet
- Temperature-WPS OfficeDocument9 pagesTemperature-WPS OfficeVhenz MapiliNo ratings yet
- Thermodynamics Module 5: Assessment Task 5: Laguna University College of Education A.Y 2020-2021Document20 pagesThermodynamics Module 5: Assessment Task 5: Laguna University College of Education A.Y 2020-2021anembam putobungbongNo ratings yet
- Heat CompleteDocument24 pagesHeat CompletePhilip MooreNo ratings yet
- Science10 Q4week1-2Document32 pagesScience10 Q4week1-2ClyzuhNo ratings yet
- Heat and ThermodynamicsDocument45 pagesHeat and ThermodynamicsbairojushivakumarNo ratings yet
- ThermodynamicsDocument78 pagesThermodynamicsHarold Jan R. TeranoNo ratings yet
- Thermal EnergyDocument29 pagesThermal EnergygerryamamooNo ratings yet
- Heat 2Document20 pagesHeat 2Sydney LezamaNo ratings yet
- QuestionsDocument2 pagesQuestionsMEOW41No ratings yet
- Temperature, Thermometry, Thermal Expansion & Gas LawsDocument50 pagesTemperature, Thermometry, Thermal Expansion & Gas LawsEmmanuel AdeyemiNo ratings yet
- Thermal ExpansionDocument18 pagesThermal ExpansionElleNo ratings yet
- Experiment 302 Heat and CalorimetryDocument2 pagesExperiment 302 Heat and CalorimetryJasmin DionisioNo ratings yet
- Chapter 10Document44 pagesChapter 10Jayaletchumi T. Sambantha MoorthyNo ratings yet
- 4Q W1 Kinetic Molecular Theory of Gases, Boyle's and Charles' LawsDocument54 pages4Q W1 Kinetic Molecular Theory of Gases, Boyle's and Charles' Lawsjia aganaNo ratings yet
- TDA301T Lesson 4 Equations of StateDocument60 pagesTDA301T Lesson 4 Equations of StateBrown Zuzu LesapoNo ratings yet
- Bses 29 ReviewerDocument8 pagesBses 29 Reviewerjerico.lapurgaNo ratings yet
- 11 ChapterDocument24 pages11 ChapterShashwat SahayNo ratings yet
- Warming The Earth and The AtmosphereDocument46 pagesWarming The Earth and The AtmosphereKylene AlimNo ratings yet
- Basic Heat 103Document48 pagesBasic Heat 103jesseamobi10No ratings yet
- Chapter 10 Thermal Properties of MatterDocument39 pagesChapter 10 Thermal Properties of MatterAnil KumarNo ratings yet
- Physics 2 1Document104 pagesPhysics 2 1Kimberly GonzalesNo ratings yet
- Changes Due To TemperatureDocument2 pagesChanges Due To TemperaturePradnyaParamitaDewiMrzNo ratings yet
- LO10 Session 08 ThermodynamicsDocument44 pagesLO10 Session 08 ThermodynamicsAbo Alphotoh GamingNo ratings yet
- Applied Physics: Lec - 1: Introduction To ThermodynamicsDocument37 pagesApplied Physics: Lec - 1: Introduction To ThermodynamicsTayyaba TanveerNo ratings yet
- Transformation by SteamDocument43 pagesTransformation by SteamLlama jennerNo ratings yet
- P103 Chapter10 TAT wk3wk4Document62 pagesP103 Chapter10 TAT wk3wk4Muhammad SaeedNo ratings yet
- Thermal Expansion of Solid Bodies by Youssef FINIANOSDocument15 pagesThermal Expansion of Solid Bodies by Youssef FINIANOSYoussefNo ratings yet
- Si UnitsDocument92 pagesSi UnitsRAVI2296No ratings yet
- Thermal Properties of Matter: Group 5Document40 pagesThermal Properties of Matter: Group 5LIYA ASKARNo ratings yet
- Website For This Class: Homework Will Be Posted at The: Phys5DDocument23 pagesWebsite For This Class: Homework Will Be Posted at The: Phys5DReeja MathewNo ratings yet
- Basic Refrigeration System - MATTERDocument10 pagesBasic Refrigeration System - MATTERCisco StarkNo ratings yet
- Physics ProjectDocument19 pagesPhysics Projectaditya varteNo ratings yet
- 1.Dr - Ahmed Samy - PhysicsDocument21 pages1.Dr - Ahmed Samy - PhysicsKhaled AhmedNo ratings yet
- C1-States of MatterDocument116 pagesC1-States of MatterGivemore MuromboNo ratings yet
- Temperature and Heat ComplteDocument62 pagesTemperature and Heat ComplteSophia BandojaNo ratings yet
- Materi Fisika AdipaDocument4 pagesMateri Fisika AdipaI Putu Adi Payana PutraNo ratings yet
- Module Thermodynamics 1-4: Colorado State UniversityDocument28 pagesModule Thermodynamics 1-4: Colorado State UniversityAsyifa Rizqi UtamiNo ratings yet
- Special Cases of The Ideal Gas LawDocument8 pagesSpecial Cases of The Ideal Gas LawJohn Benedick Beri BernalNo ratings yet
- LectureFME16Materials Science and Engineering4Document14 pagesLectureFME16Materials Science and Engineering4Cllyan ReyesNo ratings yet
- Heat and TemperatureDocument6 pagesHeat and TemperatureJavontay StewartNo ratings yet
- PHY12L E302Document4 pagesPHY12L E302Mikaella TambisNo ratings yet
- Warming The Earth and The AtmosphereDocument46 pagesWarming The Earth and The AtmosphereSalih TrexieNo ratings yet
- Temperature SensorsDocument181 pagesTemperature Sensorsbhaihello015No ratings yet
- CSEC Physics 12 Hour Crash Course Sections B and C Student Version-2Document107 pagesCSEC Physics 12 Hour Crash Course Sections B and C Student Version-2Mrs MendezNo ratings yet
- Temperature Heat Transfer: AbsorptionDocument4 pagesTemperature Heat Transfer: AbsorptionFredalyn Joy VelaqueNo ratings yet
- 4 Thermal Properties and TemperatureDocument32 pages4 Thermal Properties and Temperatureamgedalaa118No ratings yet
- Nature of Heat & Temperature 2022Document22 pagesNature of Heat & Temperature 2022Gabriella SteeleNo ratings yet
- Heat and TemperatureDocument52 pagesHeat and TemperatureAngilyn LumabasNo ratings yet
- Chapter Ten Lecture Ten Thermodynamics: TemperatureDocument16 pagesChapter Ten Lecture Ten Thermodynamics: TemperatureTony AtefNo ratings yet
- Science Script: Scene 1Document4 pagesScience Script: Scene 1Mikaela MercadoNo ratings yet
- NCERT PH 2 Thermal Properties of MatterDocument24 pagesNCERT PH 2 Thermal Properties of MatterkdsiddhantNo ratings yet
- Thermal PhysicsDocument51 pagesThermal PhysicsJerrySemuel100% (2)
- Thermodynamic Principles: Topic 1Document32 pagesThermodynamic Principles: Topic 1Jinang ShahNo ratings yet
- LF Thermal ExpansionDocument31 pagesLF Thermal ExpansionLalaine RamosNo ratings yet
- Chapter 4:physics:form 4Document10 pagesChapter 4:physics:form 4jiivi87No ratings yet
- Che 110 Exp 14Document8 pagesChe 110 Exp 14virgobabii16No ratings yet
- Temperature, Thermometry, Thermal Expansion & Gas LawsDocument52 pagesTemperature, Thermometry, Thermal Expansion & Gas LawsOlamide AyindeNo ratings yet
- Sheet - A 21 75 - Helipad Stairs DetailDocument1 pageSheet - A 21 75 - Helipad Stairs DetailMark Ronald SuaisoNo ratings yet
- Portfolio Value at Risk Commission Stock Cut Loss % 25000 250 1.19% 2000Document2 pagesPortfolio Value at Risk Commission Stock Cut Loss % 25000 250 1.19% 2000Mark Ronald SuaisoNo ratings yet
- Ch. 23 - Electric PotentialDocument93 pagesCh. 23 - Electric PotentialMark Ronald SuaisoNo ratings yet
- Gauss'S Law: Powerpoint Lectures ForDocument84 pagesGauss'S Law: Powerpoint Lectures ForMark Ronald SuaisoNo ratings yet
- Ch. 18 - Thermal Properties of MatterDocument72 pagesCh. 18 - Thermal Properties of MatterMark Ronald SuaisoNo ratings yet
- Mechanics - Theory: Variable Location For Multiple Point LoadsDocument5 pagesMechanics - Theory: Variable Location For Multiple Point LoadsMark Ronald SuaisoNo ratings yet
- Ch. 7 - Potential Energy and Energy ConservationsDocument25 pagesCh. 7 - Potential Energy and Energy ConservationsMark Ronald SuaisoNo ratings yet
- Sine and Cosine Law: Name: - DateDocument2 pagesSine and Cosine Law: Name: - DateMark Ronald SuaisoNo ratings yet
- Ch. 9 - Rotation of Rigid BodiesDocument23 pagesCh. 9 - Rotation of Rigid BodiesMark Ronald SuaisoNo ratings yet
- Ch. 8 Momentum, Impulse and CollsionsDocument29 pagesCh. 8 Momentum, Impulse and CollsionsMark Ronald SuaisoNo ratings yet
- Pig Breeding CalculatorDocument14 pagesPig Breeding CalculatorMark Ronald SuaisoNo ratings yet
- TutorMe ApplicationDocument2 pagesTutorMe ApplicationMark Ronald SuaisoNo ratings yet
- Ch. 5 - Applying Newtons LawsDocument21 pagesCh. 5 - Applying Newtons LawsMark Ronald SuaisoNo ratings yet
- Ch. 6 - Work and Kinetic EnergyDocument20 pagesCh. 6 - Work and Kinetic EnergyMark Ronald SuaisoNo ratings yet
- Ch. 3 - Motion in Two or Three DimensionsDocument33 pagesCh. 3 - Motion in Two or Three DimensionsMark Ronald SuaisoNo ratings yet
- Penrose Platter Spin Ices - Artificial Magnetic QuasicrystalsDocument38 pagesPenrose Platter Spin Ices - Artificial Magnetic QuasicrystalsEvelyn JonesNo ratings yet
- Thermotherapy PDFDocument51 pagesThermotherapy PDFRUdraNo ratings yet
- Thermodynamics Part 1 by Shobhit NirwanDocument14 pagesThermodynamics Part 1 by Shobhit NirwanKumar Okumura90% (10)
- Experiment No. 2 - Specific Heat (For 2024 - IIA APDEV)Document6 pagesExperiment No. 2 - Specific Heat (For 2024 - IIA APDEV)ladyarboleda26No ratings yet
- Adiabatic FBR DesignDocument10 pagesAdiabatic FBR DesignRana UzairNo ratings yet
- Chapter # 11 Heat & Thermodynamic Golden PointsDocument8 pagesChapter # 11 Heat & Thermodynamic Golden PointsMudasir Hussain TuriNo ratings yet
- Physics Form 4 Yearly Lesson PlanDocument22 pagesPhysics Form 4 Yearly Lesson PlanArfa Suhaida ZainNo ratings yet
- ChemDocument38 pagesChemCHLOE BEA DILLANo ratings yet
- Chapter 7 Continued Entropy: A Measure of Disorder Study Guide in PowerpointDocument53 pagesChapter 7 Continued Entropy: A Measure of Disorder Study Guide in Powerpointbrayan CortezNo ratings yet
- Access and Utilization of The Dippr Physical Property Database in Ch. E. EducationDocument12 pagesAccess and Utilization of The Dippr Physical Property Database in Ch. E. Educationingbarragan87No ratings yet
- Basic Concepts of Thermodynamics (Animation) - YouTube - EnglishDocument4 pagesBasic Concepts of Thermodynamics (Animation) - YouTube - EnglishtitojhezielanneNo ratings yet
- Energetics QuestionsDocument58 pagesEnergetics QuestionsQasim Peracha100% (1)
- MM231 LecturesDocument254 pagesMM231 LecturesMansour AbbasNo ratings yet
- Fiberfrax Blanket and Mat Products: Product Information SheetDocument8 pagesFiberfrax Blanket and Mat Products: Product Information SheetMagin Idelfonso TorreblancaNo ratings yet
- JR AIIMS S60 - NEET WET - 21 (22-11-21) SYLLABUSDocument1 pageJR AIIMS S60 - NEET WET - 21 (22-11-21) SYLLABUSCR RaghavNo ratings yet
- I.C EngineDocument154 pagesI.C EngineShafiq AhmadNo ratings yet
- Experiment 6 - Specific Heat CapacityDocument27 pagesExperiment 6 - Specific Heat CapacitydaNo ratings yet
- Topic 5 HeatDocument26 pagesTopic 5 HeatAnthonyDomNo ratings yet
- Chapter 2Document29 pagesChapter 2Bilal shahzadNo ratings yet
- 152 TOP Thermodynamics - Mechanical Engineering Multiple Choice Questions and Answers ListDocument30 pages152 TOP Thermodynamics - Mechanical Engineering Multiple Choice Questions and Answers ListSampat AgnihotriNo ratings yet
- Concise Guide To Heat Exchanger Network Design: Xian Wen NGDocument154 pagesConcise Guide To Heat Exchanger Network Design: Xian Wen NGFIRSON ANDRES SerranoNo ratings yet
- CHEN 200 Homework Problems 1: Problem 1: (3 Marks)Document2 pagesCHEN 200 Homework Problems 1: Problem 1: (3 Marks)Amy MillerNo ratings yet
- Introduction To Chemical Engineering Thermodynamics J M Smith Full ChapterDocument51 pagesIntroduction To Chemical Engineering Thermodynamics J M Smith Full Chapteragnes.baer637100% (8)