Professional Documents
Culture Documents
10.viruses As Human Pathogen
10.viruses As Human Pathogen
Uploaded by
Shimelis Teshome Ayalneh0 ratings0% found this document useful (0 votes)
11 views72 pages- Parvovirus B19 causes fifth disease in children and aplastic crisis in anemic patients. It replicates in bone marrow and destroys erythrocyte precursor cells, causing anemia.
- Papillomaviruses like HPV can cause warts and cancers like cervical cancer by binding to and deactivating tumor suppressor genes. They are transmitted through direct skin contact.
- Herpes simplex viruses 1 and 2 cause oral and genital herpes, respectively. They establish latency in nerve cells and can cause recurrent outbreaks.
Original Description:
Original Title
10.Viruses as Human Pathogen
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document- Parvovirus B19 causes fifth disease in children and aplastic crisis in anemic patients. It replicates in bone marrow and destroys erythrocyte precursor cells, causing anemia.
- Papillomaviruses like HPV can cause warts and cancers like cervical cancer by binding to and deactivating tumor suppressor genes. They are transmitted through direct skin contact.
- Herpes simplex viruses 1 and 2 cause oral and genital herpes, respectively. They establish latency in nerve cells and can cause recurrent outbreaks.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
11 views72 pages10.viruses As Human Pathogen
10.viruses As Human Pathogen
Uploaded by
Shimelis Teshome Ayalneh- Parvovirus B19 causes fifth disease in children and aplastic crisis in anemic patients. It replicates in bone marrow and destroys erythrocyte precursor cells, causing anemia.
- Papillomaviruses like HPV can cause warts and cancers like cervical cancer by binding to and deactivating tumor suppressor genes. They are transmitted through direct skin contact.
- Herpes simplex viruses 1 and 2 cause oral and genital herpes, respectively. They establish latency in nerve cells and can cause recurrent outbreaks.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 72
Recaping
• Salmonella
• Shigella
• Vibrio
02/23/2021 Shimelis Teshome (BSc MLS) 1
Viruses of Human Pathogen
Shimelis Teshome (BSc MLS)
1. DNA Viruses
1.1. Viruses with Single-Stranded DNA Genomes
A. Parvoviruses
• Parvovirus B19, is the causative virus in erythema
infectiosum (also known as “slapped cheek
syndrome” or the “fifth disease”) in children and
causes aplastic crisis in anemic patients.
• The virus also contributes to joint diseases,
embryopathies, and tissue rejection following
renal transplants.
• Diagnosis: serological (IgG and IgM) and PCR.
02/23/2021 Shimelis Teshome (BSc MLS) 3
• Parvovirus B19, the only human pathogenic
parvovirus identified to date, is capable of
autonomic replication, i.e., it requires no helper
virus.
Pathogenesis
• Parvovirus B19 replicates in the bone marrow in
erythrocyte precursor cells, which are destroyed
in the process=== anemia
• The virus also appears to cause spontaneous
abortions in early pregnancy and fetal damage in
late pregnancy (hydrops fetalis).
02/23/2021 Shimelis Teshome (BSc MLS) 4
Transmission
– Droplet infection or the fecal-oral route,
– Blood and blood products are infectious
1.2. Viruses with double-stranded DNA genomes
• Are classified in six families: papillomavirus,
polyomavirus, adenovirus, herpesvirus, poxvirus,
and hepadnavirus.
• Carcinogenic types have been found in all groups
except the poxviruses
A. Papillomaviruses
• Involved in the etiology of benign tumors such as
warts and papillomas, as well as malignancies, the
latter mainly in the genital
02/23/2021 Shimelis Teshomearea
(BSc MLS) (cervical carcinoma).
5
• Papillomaviruses possess oncogenes (E5, E6,
and E7 genes) that bind the products of tumor
suppressor genes: E6 binds the p53 gene product,
E7 the Rb gene product
• Papillomaviruses infect cells in the outer layers
of the skin and mucosa and cause various types
of warts by means of local cell proliferation
• Of all papillomavirus-caused cervical dysplasias,
50% contain human papillomavirus (HPV) 16
and 20% HPV 18
• Diagnosis:- They are detected and identified by
means of histological analysis
02/23/2021 Shimelis Teshome (BSc MLS) 6
02/23/2021 Shimelis Teshome (BSc MLS) 7
Warts Caused by Papillomaviruses
02/23/2021 Shimelis Teshome (BSc MLS) 8
02/23/2021 Shimelis Teshome (BSc MLS) 9
Papilloma Genital warts
02/23/2021 Shimelis Teshome (BSc MLS) 10
Condylomata acuminata are lesions produced by
human papillomavirus
02/23/2021 Shimelis Teshome (BSc MLS) 11
prevention.
• Since viruses are produced and accumulate in wart
tissues, papillomaviruses are transmissible by direct
contact.
• Warts can also spread from one part of the body to
another (autoinoculation).
• A certain level of prophylactic protection can be
achieved with hygienic measures.
B. Polyomaviruses
• Causes progressive multifocal leukoencephalopathy
(PML), a demyelinating disease that has become
more frequent as a sequel to HIV infections
02/23/2021 Shimelis Teshome (BSc MLS) 12
• The name polyoma refers to the ability of this
organism to produce tumors in many different
organs.
02/23/2021 Shimelis Teshome (BSc MLS) 13
C. Adenoviruses
• Adenoviruses got their name from the adenoidal
tissues (tonsils) in which they were first
identified
• They cause a wide variety of diseases.
– Upper, less frequently the lower, respiratory tract
and eye infections
– Intestinal infections
Epidemiology
• Transmission of respiratory adenoviruses is
primarily by droplet infection
02/23/2021 Shimelis Teshome (BSc MLS) 14
• Fecal-oral route, mainly by contact rather than in
water or food
• Adenoviruses are the second most frequent
diarrhea pathogen in children after rotaviruses
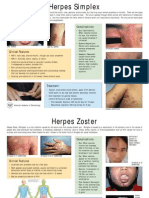
D. Herpes viruses
• Cause Gingivostomatitis to keratoconjunctivitis,
encephalitis, genital disease & neonatal infections
• Latency occur in nerve cells
• Recurrences are common
02/23/2021 Shimelis Teshome (BSc MLS) 15
Herpes simplex virus
• Two distinct viruses of HSV
• HSV-1 and HSV-2
• Mode of transmission is different
– HSV-1 is transmitted by contact with saliva
– HSV-2 is transmitted sexually / maternal to newborn
• Lesions of HSV-1and 2 are similar
• Formation of Cowdry type A intranuclear inclusion
bodies
• In these inclusion bodies, there is marginated chromatin
with multinucleated giant cells
• Cell fusion may enhance the spread of virus from one
cell to other
02/23/2021 Shimelis Teshome (BSc MLS) 16
02/23/2021 Shimelis Teshome (BSc MLS) 17
Herpes Labialis Acute herpetic
gingivostomatitis
02/23/2021 Shimelis Teshome (BSc MLS) 18
Herpesviridae- Infection and Disease
ommon Name Associated Diseases
HSV-1 Oral Herpes (cold sore), Genital Herpes
HSV-2 Genital Herpes
VZV Chicken Pox, Shingles
EBV Mononucleosis, Lymphoma, Carcinoma
CMV Mononucleosis, Retinitis, Transplant Rejection
HHV-6 Roseola infantum, Mononucleosis syndrome,
Chronic fatigue syndrome, Multiple Sclerosis?
HHV-7 Roseola infantum?, Mononucleosis syndrome?
KSHV Kaposi’s Sarcoma
02/23/2021 Shimelis Teshome (BSc MLS) 19
• The varicella-zoster virus (VZV):- causes the
primary infection chickenpox, which can then
recidivate as zoster (shingles). Acycloguanosine is
used both prophylactically and in treatment of
VZV infections
Cytomegalovirus (CMV):- infections remain
inapparent or harmless in the immunologically
healthy, but can cause generalized, fatal infections
in immunocompromised individuals.
• Ganciclovir and foscarnet are therapeutically
useful in transplantation, and particularly in AIDS
patients
02/23/2021 Shimelis Teshome (BSc MLS) 20
• The Epstein-Barr virus (EBV):- is the pathogen
in infectious mononucleosis and is also
implicated in lymphomas (including Burkitt
lymphoma) and nasopharyngeal carcinomas.
• The higher incidence of Burkitt lymphoma in
parts of Africa is attributed to a cofactor arising
from the hyperendemic presence of malaria there
– EBV exacerbates the B-cell proliferation resulting
from a malaria infection
• Lymphoproliferative diseases involving viral
replication can be treated with acyclovir and
ganciclovir
02/23/2021 Shimelis Teshome (BSc MLS) 21
The varicella zoster
viruses (VZV) persist in
the latent state in
spinal ganglia cells.
When reactivated,
they cause dermal
efflorescences in the
corresponding
dermatome
02/23/2021 Shimelis Teshome (BSc MLS) 22
Human herpesvirus 6 (HHV 6):- is the
pathogen that causes three-day fever (exanthema
subitum, roseola infantum).
Human herpesvirus 8 (HHV 8):- causes the
AIDS-associated Kaposi sarcoma.
Therapy:- Effective and well-tolerated
chemotherapeutics are available to treat herpes
simplex, varicella-zoster virus, and
cytomegalovirus (acyclovir, ganciclovir).
02/23/2021 Shimelis Teshome (BSc MLS) 23
E. Hepadnaviruses: Hepatitis B Virus and
Hepatitis D Virus
• A hepatitis B virus (HBV) infection replication)
of the liver cells results in expression of viral
antigen on the cell surface, followed by
immunological cell damage with
– Acute
– possibly fulminant
– chronic persistent or chronic aggressive hepatitis.
• The final stages can be liver cirrhosis or
hepatocellular carcinoma. A concurrent or later
superinfection by a defective,
02/23/2021 Shimelis Teshome (BSc MLS) 24
• RNA-containing and HBV-dependent hepatitis D
virus (HDV, delta agent) normally exacerbates
the clinical course. Both viruses are transmitted
in blood or body fluids, whereby even a tiny
amount of blood may be enoughto cause an
infection.
02/23/2021 Shimelis Teshome (BSc MLS) 25
2. RNA viruses
• 2.1. Viruses with Single-Stranded RNA Genomes,
Sense-Strand Orientation
• Includes picornavirus, calicivirus, togavirus,
coronavirus, flavivirus, and retrovirus
A. Picornaviruses
• The important human pathogenic genera of
picornaviruses are:
– Enteroviruses with the polioviruses (poliomyelitis),
cocksackieviruses and echoviruses.
– Parechoviruses types 1 and type 2.
– Hepatoviruses with the hepatitis A virus.
– Rhinoviruses, common cold viruses (rhinitis).
02/23/2021 Shimelis Teshome (BSc MLS) 26
• Transmission of enteroviruses, parechoviruses,
and hepatoviruses is by the fecal-oral route.
• The viruses first replicate in the intestine, from
which location they reach their target organ with
the bloodstream.
• Large numbers of inapparent infections are
typical of this group.
• Rhinoviruses are transmitted by droplet infection
and remain restricted to the upper respiratory
mucosa.
02/23/2021 Shimelis Teshome (BSc MLS) 27
02/23/2021 Shimelis Teshome (BSc MLS) 28
B. Caliciviruses
• Caliciviruses cause enteritis.
• Together with rotaviruses and adenoviruses, they are
the most frequent viral enteritis pathogens in
children, often causing minor epidemics during the
winter months (“winter vomiting disease”).
C. Flaviviruses
• Viruses in the flavivirus family (Flaviviridae) include
the genera Flavivirus, Hepacivirus, and Pestivirus.
• Flaviviruses (the prototype being the yellowfever
virus [Latin: flavus, yellow]) are transmitted by
arthropods.
02/23/2021 Shimelis Teshome (BSc MLS) 29
• They cause a biphasic infection that can have
serious consequences (hemorrhagic fever with a
high lethality rate). In southern and eastern
countries, these viruses are significant human
pathogens.
• Only one representative of this family, the
tickborne encephalitis pathogen, is encountered
in Europe.
• The hepaciviruses (hepatitis C [HCV] and
hepatitis G viruses) are not arthropodborne
02/23/2021 Shimelis Teshome (BSc MLS) 30
• HCV is transmitted mainly in blood
(transfusions, blood products, intravenous drug
use) and is a frequent cause of chronic disease
(70% of cases), including cirrhosis of the liver
and hepatocellular carcinoma.
• The hepatitis G virus (HGV) is related to HCV
and has not been characterized in detail as yet.
02/23/2021 Shimelis Teshome (BSc MLS) 31
Overview of the Most Important Flaviviruses
(arthropodborne)
02/23/2021 Shimelis Teshome (BSc MLS) 32
Overview of the Most Important Flaviviruses
(arthropodborne)
02/23/2021 Shimelis Teshome (BSc MLS) 33
D. Coronaviruses
• Infections with coronaviruses are widespread in
humans and animals.
• Human pathogens include causative agents of
rhinitislike infections and the virus of the “severe
acute respiratory syndrome” (SARS), which first
erupted in China in 2002.
• The Coronaviridae family includes several viral
species that can infect vertebrates such as dogs,
cats, cattle, pigs, rodents, and poultry.
02/23/2021 Shimelis Teshome (BSc MLS) 34
SARS
02/23/2021 Shimelis Teshome (BSc MLS) 35
Structure of the virion
SARS-CoVs are spherical enveloped, positive-stranded
RNA viruses containing the largest viral RNA genomes
known to date (27–31 kb)
Genome encoding replicase gene products and the
structural proteins containing spike (S), envelop (E),
membrane (M), and nucleocapsid (N)
S protein interacts with the cellular receptor to mediate
membrane fusion, allowing the virus to enter host cells
and is a major target for neutralizing antibodies
02/23/2021 Shimelis Teshome (BSc MLS) 36
Severe acute respiratory syndrome (SARS) is a
highly contagious respiratory disease caused by
coronavirus (CoV) named as SARS-CoV
SARS first occurred in November 2002 in
Guangdong Province of China
Has spread to 28 regions around the world in 2003
The origin of the disease was still not fully resolved
02/23/2021 Shimelis Teshome (BSc MLS) 37
The sequence data showed that the average genome
identity of the SARS-CoV-like virus from horseshoe bat
to the SARS-CoV is about 87–92%
Possible links and suspects is to look at the ecological
circles of both bats and masked palm civets
02/23/2021 Shimelis Teshome (BSc MLS) 38
A striking feature of SARS-CoV, when compared to the
other coronaviruses, is the large number of genes
encoding putative nonstructural proteins interspersed
with structural genes at the end of the genome
The function of these proteins is unknown, but they may
contribute to severe disease in infected patients and they
are nonessential for viral replication
02/23/2021 Shimelis Teshome (BSc MLS) 39
Pathogenesis
Pneumocytes and enterocytes in the respiratory system
are the primary target
SARS-CoV can also infect mucosal cells of intestines,
tubular epithelial cells of kidneys, cerebral neurons and
immune cells
It cause cell fusion
The mechanism of CPE may be attributed to Inhibition of
translation of cellular proteins and of transcription of a subset of
cellular genes
SARS-CoV proteins can affect cellular signaling pathways,
resulting in the derangement of cellular functions
Activate the expression of fibrinogen gene to induce fibrosis
02/23/2021 Shimelis Teshome (BSc MLS) 40
Epidemiology
SARS-CoV had not circulated to any significant extent in
humans prior to the outbreak in 2002 and 2003
Although animals were the original source of SARS, its
global spread occurred by human-to-human transmission
Sequences from several distinct SARS-like coronaviruses
have been amplified from horseshoe bats from Hong Kong
and several provinces in China, and 30% to 85% of this
species of bats had antibodies to a SARS-like coronavirus
The source of the 2002-2003 SARS outbreak viruses
remains unknown.
SARS-CoV infects several species of animals, including
mice, ferrets, hamsters, cats, and monkeys
02/23/2021 Shimelis Teshome (BSc MLS) 41
Clinical disease
The virus infects both upper airway and alveolar epithelial cells,
resulting in lung injury
Virus or viral products are also detected in other organs, such as
the kidney, liver, and small intestine
• SARS-CoV infection of humans nearly always resulted in a serious
lower respiratory tract illness
• Illness usually had onset at 4 to 6 days, sometimes as short as 2
days and rarely longer than 10 days after exposure
The first respiratory tract symptoms (usually a nonproductive
cough and shortness of breath)
• Patients who failed to resolve their illness often had progressive
respiratory failure that led to death within weeks, but sometimes
months, after illness onset
02/23/2021 Shimelis Teshome (BSc MLS) 42
Transmission
SARS-CoV spreads primarily through droplets
(respiratory secretions) and close person-to-person
contact
• Infectious viral particles can be excreted through
respiratory secretions, stool, urine and sweat
02/23/2021 Shimelis Teshome (BSc MLS) 43
2.2. Viruses with Single-Stranded RNA
Genomes, Antisense-Strand Orientation
• Six viral families have an antisense RNA
genome: the Orthomyxoviridae, the
Bunyaviridae, the Arenaviridae, the
Paramyxoviridae, the Rhabdoviridae, and the
Filoviridae.
• Just like all other RNA viruses, they require a
RNA-independent RNA polymerase, which
enters the cell within the viral particle in the
infective process.
02/23/2021 Shimelis Teshome (BSc MLS) 44
Rabies virus
• belongs to the family Rhabdoviridae
• Rabies is a zoonotic disease that is transmitted from
animals (particularly dogs, foxes, wolves, jackals,
monkeys and bats) to man.
• Transmitted from a bite or scratch via a puncture wound
through the skin, or through a lick on an open wound or
sore
02/23/2021 Shimelis Teshome (BSc MLS) 45
• The incubation period in man is
– usually 1–3 months, but it can be as short as 10 days
– The incubation period in the dog is usually from 14 to
60 days, but it may be much longer
Infectious period
• Once infected animals remain infectious via
infected saliva.
• Rabies is usually a fatal infection in animals but
asymptomatic infection, especially in bats, is
recognized.
– provide a long-term reservoir of infection
02/23/2021 Shimelis Teshome (BSc MLS) 46
At-risk groups
• People are at risk when
– they have not received prophylactic vaccine
– they are bitten or scratched by an infected animal, or
licked on an open wound, in a country where rabies is
prevalent.
Symptoms
• For the first 2–4 days, patients usually develop
malaise, fever, headache, sore throat and lack of
appetite.
• The virus first multiplies in the tissue around the
site of inoculation and then moves into local nerves.
02/23/2021 Shimelis Teshome (BSc MLS) 47
• Pain and tingling around the site of inoculation in the
infected limb is usually the first indication that the virus
has entered the nervous system.
• Jerky movements and increased muscle tone will follow.
• Dilation of the eye pupils and excessive secretion of
tears and saliva often occur next.
• The patient may next become anxious and frightened
when examined or disturbed
• temperature rises to 38–40C.
• Localized paralysis may follow, resulting in difficulty in
swallowing
• The fear of drinking water (hydrophobia) is very
suggestive of rabies.
02/23/2021 Shimelis Teshome (BSc MLS) 48
Laboratory diagnosis
• PCR or immunofluorescence in a reference
laboratory
• Post mortal brain biopsy can be tested for rabies
virus by PCR or immunofluorescence.
• Negri bodies are typically seen on brain
histology
Treatment
• Once symptoms have become established, there
is no effective treatment other than supportive
care.
02/23/2021 Shimelis Teshome (BSc MLS) 49
02/23/2021 Shimelis Teshome (BSc MLS) 50
Prophylaxis
• Pre-exposure prophylaxis is with 3 doses of
rabies vaccine.
• Post-exposure prophylaxis is either by 5 doses of
rabies vaccine, over a period of a month
• if the risk of rabies exposure is likely, by means
of vaccine and human anti-rabies
immunoglobulin
– half injected around the site of inoculation and half
given intramuscularly
02/23/2021 Shimelis Teshome (BSc MLS) 51
EBOLA, MARBURG VIRUS
Ebola virus was first recognized in 1976 during an outbreak in
the Ebola River valley in Zaire Africa
EBOV along with the closely related Marburg virus, cause
sporadic outbreaks of hemorrhagic fever in Central Africa, with
a mortality rate of up to 88%
Ebola Zaire and Marburg virus infection in sub-Saharan Africa
are consistently associated with case fatalities of 80–90%, and
Sudan ebolavirus 50–60%
02/23/2021 Shimelis Teshome (BSc MLS) 52
Polymorphic structure of Ebola virus
02/23/2021 Shimelis Teshome (BSc MLS) 53
Pathogenesis
GP is likely to have a role in immune suppression through
its effects on down regulation of cell surface proteins
essential for lymphocyte adhesion and antigen presentation
Soluble GP may compete for neutralizing antibodies and
inhibit neutrophil activation
Ebola virus entry depends on endosomal cathepsins,
enzymes critical for antigen presentation and release of
cathepsins may contribute to virus-induced cell damage
02/23/2021 Shimelis Teshome (BSc MLS) 54
Epidemiology
The exact origin, locations, and natural habitat (natural
reservoir) of Ebola virus remain unknown
On the basis of available evidence and the nature of similar
viruses, researchers believe that the virus is zoonotic with
four subtypes occurring in an animal host native to Africa
A similar host, most likely in the Philippines, is probably
associated with the Ebola-Reston subtype
MARV has apparently been contracted in forested and
derived areas of Kenya, Uganda, Zimbabwe, DRC, and
recently Angola
02/23/2021 Shimelis Teshome (BSc MLS) 55
Clinical symptoms of Ebola virus
With the exception of Reston Ebola virus, which does not
appear to be pathogenic to humans, all the Filoviruses appear to
produce a similar illness
Early symptoms
Arthritis ,low-back pain, chills ,diarrhea, Fatigue, fever,
headache ,Malaise ,nausea sore throat ,and vomiting
Late symptoms include:
• Bleeding from eyes, ears, nose ,mouth and rectum
(gastrointestinal bleeding)
• Depression
• Inflammation of eye and genitals
• Increased feeling of pain in skin
• Rash over the entire body that often contains blood
(hemorrhagic)
02/23/2021 Shimelis Teshome (BSc MLS) 56
02/23/2021 Shimelis Teshome (BSc MLS) 57
Transmission of Ebola virus
The manner in which the virus first appears in a human at
the start of an outbreak has not been determined
Recent studies have found that fruit bats may support
replication of Ebola virus, indicating that these animals
may be involved in the life cycle of the virus
Human infections usually occur after direct contact with
virus in dead or infected people or wildlife, with
subsequent person-to-person transmission
Airborne particles (aerosols) spread has not been
documented among humans in a real-world setting, such
as a hospital or household
02/23/2021 Shimelis Teshome (BSc MLS) 58
HIV
Origin and Distribution
• HIV was emerged following cross species transmission from
non-human primate.
• Cross-species transmitted simian immunodeficiency viruses
to human hosts
• Adapted to allow human to human transmission
• According to ICTV the species of HIV1 and HIV2 were
classified under the family of Retroviridae, sub family of
orthoretrovirinae, and genera of lentivirus
• while there order was not assigned yet
02/23/2021 Shimelis Teshome (BSc MLS) 59
Figure 3: Origins of human HIV viruses
02/23/2021 Shimelis Teshome (BSc MLS) 60
• HIV has grouped into two namely HIV-1 and HIV-2
• The worldwide main agent of AIDS is HIV-1
• HIV-2 is restricted to some regions of Western and Central
Africa.
• HIV-2 prevalence is decreasing and HIV-1 becomes
predominant in West Africa
• Dual infections with HIV-1 and HIV-2 have been frequently
observed where both viruses co-circulate
• but today no recombinant virus between HIV-1 and 2 has
been documented yet
02/23/2021 Shimelis Teshome (BSc MLS) 61
• Both differ based on clinical disease progression
• In HIV-2 disease progression occurs at higher CD4 counts
and at lower plasma viral loads
• More frequent occurrence of circulating recombinant
forms (CRF) among HIV 1 than HIV 2.
• Among HIV 1 until now 88 CRF were identified while
only one CRF was identified that belongs HIV 2
02/23/2021 Shimelis Teshome (BSc MLS) 62
• HIV-1 variants are classified into three major
phylogenetic groups: group M (main), group O (outlier),
and group N (non-M/non-O).
• Recently a group P was isolated in Cameroonian woman
• group P is derived from SIVgor
• Group M is responsible for the majority of infections in
the worldwide HIV-1 epidemic
– F urther subdivided into 10 recognized phylogenetic
subtypes, or clades (A to K)
02/23/2021 Shimelis Teshome (BSc MLS) 63
• Subtype A has been responsible for 80% of the HIV-
infections in Western Africa, and for 30% in Eastern
Africa
• Subtype B has been the main epidemic component in the
Western Europe (60%),
• Subtype C represents 60% of HIV infections worldwide,
predominantly in East Africa and South Asia
02/23/2021 Shimelis Teshome (BSc MLS) 64
• Gross Structure of HIV Virions
• HIV exhibit cone shaped capsid
• HIV is enveloped by a lipid bilayer, derived from host
cell
Figure 6: Schematic illustration of HIV virion
02/23/2021 Shimelis Teshome (BSc MLS) 65
HIV Pathogenesis
Target Cells and Mechanism of Dissemination
• HIV infection can occur through mucosal surfaces, even in
the absence of mucosal disruption.
• In infant as mucosal surface is thinner the virus can easily
establish itself
• Routes of HIV entry include DC, epithelial cells, and
microfold (M) cells
• In the early phases HIV preferentially targets
CCR5+CD4+memory Shimelis
02/23/2021
T lymphocytes
Teshome (BSc MLS)
in GIT 66
• HIV 1 pathogenesis associated with macrophages is
among the challenge in HIV clearance and cure
– as macrophage act as major reservoirs for HIV-1 in
tissues of the body
• The streaking feature of HIV pathogenesis a change in
tropism after infection is established
• The tropism switch involves switching from using
CCR5 to CXCR4
02/23/2021 Shimelis Teshome (BSc MLS) 67
HIV Persistence and Eradication
Antiretroviral Therapy
• Used for HIV prevention
• It also dramatically suppresses viral replication and
reduces the plasma HIV-1 viral load
• Combination therapy using three antiretroviral agents
directed against at least two targets
• ART act based on the virus replication to halt the viral
natural history
02/23/2021 Shimelis Teshome (BSc MLS) 68
Figure 14:Targets of antiretroviral drugs in the HIV life cycle
02/23/2021 Shimelis Teshome (BSc MLS) 69
HIV Latency, Reservoirs, and Potential cure
• antiretroviral therapy is unable to cure HIV and lifelong
treatment is needed
• HIV can persist in patients on ART because of
– resting memory T cells that are long lived
– latently infected
– Residual replication in some individuals
02/23/2021 Shimelis Teshome (BSc MLS) 70
HIV Latency
• Is integration of HIV DNA into the host genome in the
absence of virus production
• It is the major obstacle towards HIV-1 eradication
• Occurs in memory and in naïve T cells, monocytes,
macrophages and astrocytes
• invisible to the host immune system and unaffected by
existing antiviral drugs.
• Rebound of viremia and recovery of systemic infection that
follows interruption ART
02/23/2021 Shimelis Teshome (BSc MLS) 71
02/23/2021 Shimelis Teshome (BSc MLS) 72
You might also like
- Summary of Georgia Immunization Requirements For Child Care & School AttendanceDocument1 pageSummary of Georgia Immunization Requirements For Child Care & School AttendanceWSB-TV Assignment DeskNo ratings yet
- Lec 2 VirologyDocument11 pagesLec 2 VirologyAbdullah EmadNo ratings yet
- Herpesviruses S MunsakaDocument20 pagesHerpesviruses S Munsakamulengamordecai92No ratings yet
- HERPESVIRUSES, PARVO 2021 Students - KopieDocument38 pagesHERPESVIRUSES, PARVO 2021 Students - KopieMr.FantasthiccNo ratings yet
- Herpes VirusesDocument6 pagesHerpes VirusesAlya Putri KhairaniNo ratings yet
- DNA Enveloped Viruses 2Document13 pagesDNA Enveloped Viruses 2Tamara ElyasNo ratings yet
- Human Herpes VirusesDocument48 pagesHuman Herpes Virusesfiea241089100% (1)
- HerpesviridaeDocument42 pagesHerpesviridaeM. RamazaliNo ratings yet
- Herpes VirusesDocument26 pagesHerpes VirusesUmar'Farouq OniNo ratings yet
- Opportunistic Viral InfectionsDocument40 pagesOpportunistic Viral InfectionsjohndemoNo ratings yet
- Virology Lecture 7 Viral DiseseDocument21 pagesVirology Lecture 7 Viral Diseseao868598No ratings yet
- 21 - Virology I (DNA) LectureDocument47 pages21 - Virology I (DNA) LectureSamqureshiNo ratings yet
- 5 Herpes VirusesDocument5 pages5 Herpes VirusesTᕼE FᗩᗪEᗪ ᔕOᑌᒪNo ratings yet
- Herpesviruses: Department of Microbiology and ParasitologyDocument96 pagesHerpesviruses: Department of Microbiology and Parasitology2013SecBNo ratings yet
- Viral Infections of The Genitourinary TractDocument11 pagesViral Infections of The Genitourinary Tract46nv7gphxzNo ratings yet
- DNA Viruses IIDocument4 pagesDNA Viruses IIkep1313No ratings yet
- VirologyDocument5 pagesVirologyAmnah ✨No ratings yet
- Herpes Viruses LectureDocument46 pagesHerpes Viruses Lectureapi-19969058100% (1)
- Medt 23 The Viruses Midyr 2021Document25 pagesMedt 23 The Viruses Midyr 2021Shania DawnNo ratings yet
- Chapter 13 Viral PathogenesisDocument68 pagesChapter 13 Viral PathogenesisKelly WareNo ratings yet
- Dna VirusesDocument3 pagesDna VirusesAshamdeep AntaalNo ratings yet
- HerpesvirusesDocument54 pagesHerpesvirusessameera ruffaiNo ratings yet
- Herpesvirida E& Adenoviridae: By: MJ Briones Bsn-IiDocument93 pagesHerpesvirida E& Adenoviridae: By: MJ Briones Bsn-IiMj BrionesNo ratings yet
- Herpes Simplex VirusDocument4 pagesHerpes Simplex VirusUlfat IqbalNo ratings yet
- HerpesvirusesDocument25 pagesHerpesvirusesHoor Ul Ain RounaqNo ratings yet
- Human HerpesDocument25 pagesHuman HerpesKannan KrishnamurthyNo ratings yet
- Microbiology Short ReviewDocument33 pagesMicrobiology Short ReviewMoNiruzzaman MoNirNo ratings yet
- VirologyDocument24 pagesVirologyRohama Qubra 279No ratings yet
- DNA VirusesDocument62 pagesDNA VirusesGabriella CrooksNo ratings yet
- Cmv&epstein-Barr VirusDocument8 pagesCmv&epstein-Barr VirusAtheer AlabdyNo ratings yet
- PAPOVIRUSESDocument35 pagesPAPOVIRUSESFrancis MakanyaNo ratings yet
- Herpes VirusesDocument71 pagesHerpes VirusesAyioKunNo ratings yet
- Herpes Virus FinalDocument14 pagesHerpes Virus Finalshoiabmalik002No ratings yet
- DNA VirusesDocument99 pagesDNA VirusesCourtny Lenz Maygay GapaNo ratings yet
- Cardiovascular System VirologyDocument18 pagesCardiovascular System Virology46nv7gphxzNo ratings yet
- Foundations in Microbiology: TalaroDocument50 pagesFoundations in Microbiology: Talaromertx013No ratings yet
- Clinical VirologyDocument31 pagesClinical VirologySally ElhadadNo ratings yet
- HerpervirusesDocument66 pagesHerperviruseskomalsaharan2001No ratings yet
- Viral InfectionsDocument103 pagesViral InfectionsAkash Anilkumar MaliniNo ratings yet
- Viral Diseases 45Document27 pagesViral Diseases 45Mujtaba AlsalehNo ratings yet
- K26. Infeksi Virus Pada KulitDocument48 pagesK26. Infeksi Virus Pada KulitFajar Cristianta GintingNo ratings yet
- Herpes VirusDocument80 pagesHerpes VirusngussutsegaNo ratings yet
- VirusDocument51 pagesVirusBatool SherbiniNo ratings yet
- Microbiology PathologyDocument114 pagesMicrobiology Pathologykhaleelkhamjan100% (1)
- Herpes Viruses 2Document36 pagesHerpes Viruses 2الطاهر زروقNo ratings yet
- Herpetic DiseasesDocument18 pagesHerpetic DiseasesKrishna VadodariyaNo ratings yet
- Oral Viral Infections Diagnosis and Management PDFDocument13 pagesOral Viral Infections Diagnosis and Management PDFWesley RodriguesNo ratings yet
- At-A-Glance: Human Papillomavirus InfectionsDocument26 pagesAt-A-Glance: Human Papillomavirus Infectionsketty putriNo ratings yet
- Oral Viral Infections Diagnosis and ManagementDocument13 pagesOral Viral Infections Diagnosis and ManagementhunarsandhuNo ratings yet
- OMIC 311 Lecture 17 Human Papilloma, Herpes, Hepatits VirusDocument51 pagesOMIC 311 Lecture 17 Human Papilloma, Herpes, Hepatits VirusMaryam XANo ratings yet
- Acyclovir Resistance in Herpes Simplex VirusesDocument21 pagesAcyclovir Resistance in Herpes Simplex VirusesZaenab AzzahraNo ratings yet
- Lecture 5 - Papilloma, Polyoma, & Parvo VirusesDocument28 pagesLecture 5 - Papilloma, Polyoma, & Parvo VirusesJeanPaule JoumaaNo ratings yet
- HIV ENT ManifestationDocument33 pagesHIV ENT ManifestationAhmad fayazNo ratings yet
- Viral Infections of The SkinDocument59 pagesViral Infections of The SkinetNo ratings yet
- Purnomo Hadi: FK UNDIP - SemarangDocument36 pagesPurnomo Hadi: FK UNDIP - SemarangandreasNo ratings yet
- Myco Viro Hand Out Batch1Document16 pagesMyco Viro Hand Out Batch1Mark jay LlanoNo ratings yet
- Dna Gruop Virus: Clinical ImportanceDocument3 pagesDna Gruop Virus: Clinical ImportanceAngela HabaradasNo ratings yet
- Feline Immunodeficiency Virus: From Diagnosis to Well-being for Cats with FIVFrom EverandFeline Immunodeficiency Virus: From Diagnosis to Well-being for Cats with FIVNo ratings yet
- Vaccinated: From Cowpox to mRNA, the Remarkable Story of VaccinesFrom EverandVaccinated: From Cowpox to mRNA, the Remarkable Story of VaccinesRating: 4 out of 5 stars4/5 (39)
- Systemic MycologyDocument52 pagesSystemic MycologyShimelis Teshome AyalnehNo ratings yet
- Systemic Bacteriology 2Document43 pagesSystemic Bacteriology 2Shimelis Teshome AyalnehNo ratings yet
- ParasitologyDocument107 pagesParasitologyShimelis Teshome AyalnehNo ratings yet
- 7.systemic BacteriologyDocument88 pages7.systemic BacteriologyShimelis Teshome AyalnehNo ratings yet
- Immunology: Shimelis Teshome (BSC MLS)Document33 pagesImmunology: Shimelis Teshome (BSC MLS)Shimelis Teshome AyalnehNo ratings yet
- Adaptive Immunity: Shimelis Teshome (BSC MLS)Document31 pagesAdaptive Immunity: Shimelis Teshome (BSC MLS)Shimelis Teshome AyalnehNo ratings yet
- Bacterial GrowthDocument48 pagesBacterial GrowthShimelis Teshome AyalnehNo ratings yet
- Microbial GeneticsDocument42 pagesMicrobial GeneticsShimelis Teshome AyalnehNo ratings yet
- General MycologyDocument28 pagesGeneral MycologyShimelis Teshome AyalnehNo ratings yet
- Final ExamDocument4 pagesFinal ExamShimelis Teshome AyalnehNo ratings yet
- Intro and General BacteriologyDocument74 pagesIntro and General BacteriologyShimelis Teshome AyalnehNo ratings yet
- Hematology and Nephrology Color PlatesDocument4 pagesHematology and Nephrology Color PlatesShimelis Teshome AyalnehNo ratings yet
- Malaria Prasite Morphology: Prepared By: Shimelis Teshome/ Laboratory TechnologistDocument13 pagesMalaria Prasite Morphology: Prepared By: Shimelis Teshome/ Laboratory TechnologistShimelis Teshome AyalnehNo ratings yet
- Muhammad Rizky AsfaradaDocument7 pagesMuhammad Rizky AsfaradaAdi Indra WBNo ratings yet
- Boalan Es Health Condition 2023 FinalDocument2 pagesBoalan Es Health Condition 2023 FinalMely DelacruzNo ratings yet
- Public Health Approach (Dengue)Document10 pagesPublic Health Approach (Dengue)Joanna CamayangNo ratings yet
- Jurnal 3 NasionalDocument20 pagesJurnal 3 NasionalFajri WengkengNo ratings yet
- Nipah Virus A Deadly VirusDocument2 pagesNipah Virus A Deadly VirusTasyaNo ratings yet
- RockerDocument2 pagesRockerwan hseNo ratings yet
- IHSA-Skin Conditions ManualDocument31 pagesIHSA-Skin Conditions ManualnmillerNo ratings yet
- ADA COVID-19 Daily Screening LogDocument8 pagesADA COVID-19 Daily Screening LogMadalinaNo ratings yet
- Virus ZikaDocument2 pagesVirus Zikainta hestyaNo ratings yet
- Ebola Hemorrhagic Fever HandoutDocument3 pagesEbola Hemorrhagic Fever Handoutapi-283376958No ratings yet
- Deteksi PUS RestiDocument45 pagesDeteksi PUS RestiPuskesmas LasemNo ratings yet
- Rhino VirusesDocument20 pagesRhino VirusesstudymedicNo ratings yet
- EPI Manual For BHS 2017Document344 pagesEPI Manual For BHS 2017samuel winphyoeNo ratings yet
- The Great Influenza of 1918Document2 pagesThe Great Influenza of 1918Lois Stanley100% (1)
- HerpesDocument2 pagesHerpesSyafruddin GBNo ratings yet
- Epidemiology RabiesDocument38 pagesEpidemiology RabiesGanet PramukantoroNo ratings yet
- (MICROBIO) Viruses Reviewer (Edited Ver)Document33 pages(MICROBIO) Viruses Reviewer (Edited Ver)CRUZ Jill EraNo ratings yet
- Congenital RubellaDocument15 pagesCongenital RubellaShanaz AlvikhaNo ratings yet
- Narrative ReportDocument5 pagesNarrative Reportvanessa mananzanNo ratings yet
- Herpes Zoster OticusDocument12 pagesHerpes Zoster Oticusraghad.bassalNo ratings yet
- HIV - AIDS PresentationDocument17 pagesHIV - AIDS PresentationphilNo ratings yet
- 12 - Epstein Barr Virus (EBV)Document20 pages12 - Epstein Barr Virus (EBV)Lusiana T. Sipil UnsulbarNo ratings yet
- 2nd MAPEH 4Document2 pages2nd MAPEH 4Edmar MejiaNo ratings yet
- Informed Consent For Hiv Testing Doh-Nec Form A 2014Document2 pagesInformed Consent For Hiv Testing Doh-Nec Form A 2014smoothp3akNo ratings yet
- Dengue Fever Textbook DiscussionDocument2 pagesDengue Fever Textbook DiscussionKeyyth OirelNo ratings yet
- AS Shona TopicsDocument2 pagesAS Shona TopicsCyborg SurgeonNo ratings yet
- Daftar Diagnosa Penyakit Berdasarkan Icd-10: Poli Dalam Poli ParuDocument4 pagesDaftar Diagnosa Penyakit Berdasarkan Icd-10: Poli Dalam Poli ParuNannda SuccindaaNo ratings yet
- Form SKDRDocument123 pagesForm SKDRMochammad SyamsNo ratings yet
- Incubation Period: Jannah Marie A. Dimaporo - BIO030 (Section Uu)Document2 pagesIncubation Period: Jannah Marie A. Dimaporo - BIO030 (Section Uu)Jannah Marie A. DimaporoNo ratings yet