Professional Documents
Culture Documents
Preview: The Nucleus Nuclear Decay Nuclear Reactions Particle Physics
Preview: The Nucleus Nuclear Decay Nuclear Reactions Particle Physics
Uploaded by
mahsan abbasOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Preview: The Nucleus Nuclear Decay Nuclear Reactions Particle Physics
Preview: The Nucleus Nuclear Decay Nuclear Reactions Particle Physics
Uploaded by
mahsan abbasCopyright:
Available Formats
Subatomic Physics Section 1
Preview
Section 1 The Nucleus
Section 2 Nuclear Decay
Section 3 Nuclear Reactions
Section 4 Particle Physics
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
What do you think?
• What holds a nucleus together?
• What particles exist within the nucleus?
• What force(s) exist between these particles?
• Are these forces attractive or repulsive?
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
The Nucleus
• The chemical symbol for an
element is written like the one
shown to the left. What
information is provided by this
symbol?
– The atomic number (Z) or
number of protons is 13.
– The mass number (A) or number
of protons + neutrons is 27.
– The number of neutrons (N) is
14 (27 – 13).
– The element is aluminum.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Isotopes
• Isotopes are atoms of the same element with
different atomic masses.
– The number of neutrons is different.
• Most carbon nuclei have 6 protons and 6
neutrons and an atomic mass of 12.
– Called carbon-12
– Others have 5 neutrons (carbon-11), 7 neutrons
(carbon-13), or 8 neutrons (carbon-14).
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Isotopes
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Nuclear Mass
• The density of the nucleus is
approximately 2.3 1017 kg/m3.
• Mass is measured in unified
mass units (u).
– 1 u is one-twelfth the mass of one
atom of carbon-12.
• 1 u = 1.6605 10-27 kg
• Protons and neutrons each
have a mass of approximately
1 u.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Nuclear Mass
• Find the energy equivalent of 1 u in both J and eV.
(For c, use the value 2.9979 108 m/s.)
– Answers:
• 1.4924 10-44 J, 931.47 106 eV or 931.47 MeV
• With more significant figures, 1 u = 931.49 MeV.
• The mass of subatomic particles is often expressed in
MeV.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Nuclear Mass
• This table provides the mass and rest energy of atomic
particles in kilograms, unified mass units, and MeV.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Nuclear Stability
• What type of electric force would
exist in the nucleus shown?
– Protons would repel other protons
very strongly because the distance
between them is small.
– Neutrons would produce no forces.
• What holds the nucleus together?
– A force called the strong force:
a powerful attractive force between
all particles in the nucleus
• Does not depend on charge
• Exists only over a very short range
© Houghton Mifflin Harcourt Publishing Company
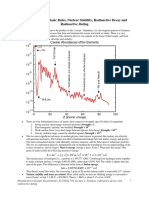
Subatomic Physics Section 1
Nuclear Stability
• As more protons are added to the nucleus, more
repulsion exists.
– Larger and larger nuclei require more neutrons, and
more strong force, to maintain stability.
• Look at a periodic table to find out which
elements have approximately a 1:1 ratio between
neutrons and protons, and which elements have
the highest ratio of neutrons to protons.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Nuclear Stability and Ratio of Neutrons and
Protons
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Binding Energy
• The nucleons (protons and neutrons) have a greater
mass when unbound than they do after binding to form a
nucleus.
– Called binding energy
– This energy is released when the binding occurs, and must be
absorbed to separate the nucleons.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Classroom Practice Problem
• The mass of the individual particles in an atom is
the mass of the protons, neutrons, and
electrons.
– For the mass of the protons and electrons combined,
simply multiply the atomic number times the mass of a
hydrogen atom (1 electron bound to 1 proton).
• Find the binding energy (in u and MeV) for a
helium atom with two protons and two neutrons.
The atomic mass of helium-4 is 4.002602 u.
– Answer: 0.030378 u or 28.297 MeV
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 1
Now what do you think?
• What holds a nucleus together?
• What particles exist within the nucleus?
• What forces exist between these particles?
• Are these forces attractive or repulsive?
• What happens to each of these forces when the
particles are farther and farther apart?
• What is meant by the term binding energy?
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
What do you think?
• Often scientists use radioactive carbon
dating to determine the age of fossils.
• What does the term radioactive mean?
• Are all atoms radioactive?
• If not, how are radioactive atoms different from those
that are not radioactive?
• How can radioactivity be used to determine the
age of a fossil?
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Nuclear Decay
• When nuclei are unstable, particles and
photons are emitted.
– The process is called radioactivity.
– It occurs because the nucleus has too many or
too few neutrons.
– Three types of radiation can occur:
• Alpha
• Beta
• Gamma
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Alpha Decay ()
• An alpha particle (2 protons and 2 neutrons) is emitted
from the nucleus.
particles are helium-4 nuclei.
– A new element is formed by alpha decay.
– Example of alpha decay:
238
92 U
234
90
4
Th + He
2
• The uranium atom has changed into a thorium atom by
ejecting an alpha particle.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Radioactive Decay
• These rules are used to determine the daughter nucleus
when a parent nucleus decays.
• Note how these rules apply in the alpha decay of
uranium-238:
– 238 = 234 + 4
– 92 = 90 + 2
238
92 U
234 4
Th + He
90 2
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Beta Decay
• An electron or positron is emitted from the
nucleus.
– A positron is the same as an electron but with an
opposite charge.
– A positron is the antiparticle of an electron.
• Since there are no electrons or positrons in the
nucleus, how can beta decay occur?
– A neutron is transformed into a proton and an electron,
and then the electron is ejected.
– A proton is transformed into a positron and a neutron,
and then the positron is ejected.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Beta Decay
• It was discovered that, during beta decay, momentum and
energy were not conserved.
– The ejected electron did not have as much forward momentum as
the recoiling nucleus.
• In 1930, Wolfgang Pauli proposed the existence of a
particle that was not detectable at the time.
• In 1956, Pauli’s neutrino () was detected.
• The neutrino and its antiparticle, the antrineutrino (), are
emitted during beta decay.
– Electrons are accompanied by antineutrinos.
– Positrons are accompanied by neutrinos.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Beta Decay
• What new element is formed by the beta decay
of carbon-14?
14
6 N + e +
C 14
7
0
-1
• The new element is nitrogen-14.
• The electron is shown with a mass number of
zero and an atomic number of -1.
– The total of the mass numbers and atomic numbers
are still equal.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Gamma Decay
• During alpha and beta decay, the nucleons left
behind are often in an excited state.
• When returning to ground state, the nucleus
emits electromagnetic radiation in the form of a
gamma ray.
• The nucleus remains unchanged except for its
energy state.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Types of Radioactive Decay
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Alpha, Beta, and Gamma Radiation
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Nuclear Decay Series
• During nuclear decay, the daughter may be
unstable as well, causing further decays.
• What element would be formed by thorium-232
undergoing 6 alpha and 4 beta decays?
– Answer: lead-208
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Classroom Practice Problems
• Find the missing item (X) in these reactions:
228
88 X + -10 e+
Ra
– Answer: 228
88 Ra
228
89 Ac + e +
0
-1
220
86 Rn
216
84 Po + X
– Answer: 220
86 Rn
216
84 Po + 42 He
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Measuring Nuclear Decay
• The rate of decay is different for each nucleus.
N/t = -N
– N is the number of nuclei, t is the time, and is the
decay constant.
differs for every element.
– The rate of decay is called the activity.
– The negative sign occurs because the number of
nuclei is decreasing.
– SI unit: becquerel (Bq) or decays/s
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Half-Life
• Half life is the time required for half of the nuclei to
decay.
– Half-lives can be very short (nanoseconds) or very long (millions
of years).
• Half-life is inversely related to the decay constant.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Half-Life
• Carbon-14 is radioactive with a
half-life of 5715 years.
• The figure shows a decay curve
for carbon-14.
– Does the total number of nuclei
change?
• No
– How much time has passed at
T1/2?
• 5715 years
– How much time has passed at
2T1/2?
• 11 430 years
– How many blue circles will there
be at 3T1/2 ?
• one
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Radioactive Carbon Dating
• All living things have about the same ratio of
carbon-14 to carbon-12.
– Carbon-14 is radioactive, and carbon-12 is not.
– After death, the ratio drops because the carbon-14
decays into nitrogen-14, while the carbon-12 is stable
and remains.
– When the ratio is half the starting ratio, 5715 years
have passed since death occurred.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Classroom Practice Problems
• A sample of barium-144 contains
5.0 10 9 atoms. The half-life is about 12 s.
– What is the decay constant of barium-144?
– How many atoms would remain after 12 s?
– How many atoms would remain after 24 s?
– How many atoms would remain after 36 s?
• Answers:
– 0.058 s-1
– 2.5 109 atoms, 1.2 109 atoms, 6.2 108 atoms
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Half-Life
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 2
Now what do you think?
• Often scientists use radioactive carbon
dating to determine the age of fossils.
• What does the term radioactive mean?
• Are all atoms radioactive?
• If not, how are the radioactive atoms different from
those that are not radioactive?
• How can radioactivity be used to determine the
age of a fossil?
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
What do you think?
• Nuclear power and nuclear weapons are
important and frequently-discussed issues in the
world today.
• How does a nuclear reactor produce energy?
• What is nuclear about it?
• What problems are associated with nuclear power?
• Do atomic bombs and hydrogen bombs differ in the
way they produce energy?
• If so, how are they different?
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Nuclear Changes
• For nuclear changes to
occur naturally, energy
must be released.
– Binding energy must
increase.
– Lighter elements must
combine, and heavier
elements must reduce in
size.
– The greatest stability is for
atoms with mass numbers
between 50 and 60.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Fission
• Fission occurs when a large nucleus absorbs a
neutron and splits into two or more smaller
nuclei.
• Example of fission:
1
0 n+ 235
92 U
236
92 U *
X + Y + neutrons
– It only occurs for heavy atoms.
– The * indicates an an unstable state that lasts for
about a trillionth of a second.
– X and Y can be different combinations of atoms that
have a total atomic number of 92.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Fission
1
0 n+ 235
92 U
140
56 Ba + 93
36
1
Kr + 3 n
0
• A typical fission reaction is shown above.
• The products, Ba and Kr, have more binding energy than
the uranium.
• As a result, energy is released.
– Each fission yields about 100 million times the energy released
when burning a molecule of gasoline.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Chain Reaction
• On the average, 2.5
neutrons are released
with each fission.
• These neutrons are
then absorbed and
cause more fissions.
– A chain reaction
occurs.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Nuclear Fission
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Nuclear Reactors
• Reactors manage the fission rate by inserting
control rods to absorb some of the neutrons.
• Nuclear power plants and navy vessels use
fission reactions as an energy source.
– Reactors produce radioactive waste, and disposal is
one difficulty.
– Presently 20% of the U.S. electric power is generated
by nuclear reactors.
• Atomic bombs use uncontrolled fission.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Fusion
• Light elements can combine and release energy
as well.
– Hydrogen atoms have less binding energy per
nucleon than helium atoms.
• Fusion is the source of a star’s energy.
– Hydrogen atoms fuse to form helium atoms.
– Much energy is released with each fusion.
• Hydrogen bombs use uncontrolled fusion.
– First tested in 1952 but never used in war
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Fusion as an Energy Source
• Fusion reactors are being developed.
• Advantages of fusion reactors:
– The fuel source, hydrogen from water, is cheap.
– The products of fusion are clean and are not
radioactive.
• Disadvantages of fusion:
– It requires extremely high temperatures of roughly
108 K to force atoms to fuse.
– It is difficult to keep the hydrogen atoms contained at
this temperature.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Nuclear Fusion
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 3
Now what do you think?
• Nuclear power and nuclear weapons are
important and frequently-discussed issues in the
world today.
– How does a nuclear reactor produce energy?
• What is nuclear about it?
– What problems are associated with nuclear power?
– Do atomic bombs and hydrogen bombs differ in the
way they produce energy?
• If so, how are they different?
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
What do you think?
• When the idea of the atom was first conceived,
it was thought to be a fundamental particle,
indivisible and indestructible. We now know
differently.
• List every particle you can think of that is smaller
than an atom.
• If you know the properties of these particles, list them as
well.
• Which of the particles on your list are fundamental?
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Fundamental Forces
• There are four fundamental interactions or forces in
nature:
– strong
– electromagnetic
– weak
– gravitational
• They exert force using the
exchange of mediating
particles.
– Photons are the mediating
particle exchanged between
electrons.
• This causes repulsion.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Fundamental Forces
• Strong force
– Holds protons and neutrons together in the nucleus
• Electromagnetic force
– Creates forces between charged particles
– Holds atoms and molecules together
• Weak force
– A nuclear force that controls radioactive decay
• Gravitational force
– The weakest force
– Gravitons (the mediating particle) not yet discovered
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Fundamental Forces
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Classification of Particles
• All particles are classified as
leptons, hadrons, or mediating
particles.
– Over 300 particles are
known.
• Leptons are thought to be
fundamental.
– Electrons are leptons.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Classification of Particles
• Hadrons are composed of
smaller particles called
quarks.
– Quarks are thought to be
fundamental.
– Protons and neutrons are
hadrons.
• Two types of hadrons:
baryons and mesons
• Hadrons interact through all
four of the fundamental
forces, while leptons do not
participate in strong force
interactions.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Classification of Particles
• Protons and neutrons are
baryons.
• What combination of up and
down quarks would make a
proton and a neutron?
– Two up quarks (+4/3) and one
down quark (-1/3) gives a
proton a charge of +1.
– One up quark (+2/3) and two
down quarks (-2/3) gives a
neutron a charge of zero.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Combinations of Quarks
• Baryons and mesons are distinguished by their internal
structure.
• The particles above are a proton, a neutron, a pion, and
a kaon.
• Mesons are unstable, and are not constituents of
everyday matter.
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
The Standard Model of Particle Physics
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
The Standard Model
• The Standard Model is the current model used in particle physics.
• How many fundamental particles are there in the standard model?
– Six quarks, six leptons, and an antiparticle for each (24 total)
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Evolution of the Four Forces
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Quarks and their Charges
Click below to watch the Visual Concept.
Visual Concept
© Houghton Mifflin Harcourt Publishing Company
Subatomic Physics Section 4
Now what do you think?
• When the idea of the atom was first conceived, it
was thought to be a fundamental particle,
indivisible and indestructible. We now know
differently.
• List every particle you can think of that is smaller than
an atom.
• If you know the properties of these particles, list them as well.
• Which of the particles on your list are fundamental?
• What are the four fundamental forces and what
particle mediates each?
© Houghton Mifflin Harcourt Publishing Company
You might also like
- Pneumatic Reciprocating Powere Hacksaw Machine - Docx FinalDocument11 pagesPneumatic Reciprocating Powere Hacksaw Machine - Docx FinalARVIND DARLING100% (2)
- MEP003A Manual For 10kW Military Diesel GeneratorDocument243 pagesMEP003A Manual For 10kW Military Diesel GeneratorGreenMountainGenerators100% (1)
- Forces: AQA 2016 Physics Topic 5Document104 pagesForces: AQA 2016 Physics Topic 5mahsan abbasNo ratings yet
- 9 Electricity Test - CircuitsDocument5 pages9 Electricity Test - CircuitsRahmonGreen100% (1)
- Risk Assessment FormDocument3 pagesRisk Assessment FormThomas100% (1)
- Nuclei Chapter 12 Class12 PhysicsDocument30 pagesNuclei Chapter 12 Class12 Physicsharikeshsingh45497No ratings yet
- Unit I Nuclear Structure & RadioactivityDocument66 pagesUnit I Nuclear Structure & RadioactivityAnupriya AnuNo ratings yet
- G12-Phy-CH 26 W3D1Document13 pagesG12-Phy-CH 26 W3D1ZARMINA SAROORNo ratings yet
- Lec - Nuclear-PhysicsDocument49 pagesLec - Nuclear-PhysicsNovi Ann Yap LiboonNo ratings yet
- Chemistry For Engineers 1 Energy Topic 04 Nuclear Chemistry 1Document6 pagesChemistry For Engineers 1 Energy Topic 04 Nuclear Chemistry 1Kristine AlcantaraNo ratings yet
- Nuclear PowerplantDocument35 pagesNuclear Powerplantasuf skakNo ratings yet
- Atomic NucleusDocument10 pagesAtomic NucleusKristiyan GeorgievNo ratings yet
- Final CBRNDocument28 pagesFinal CBRNRavindra JoshiNo ratings yet
- Lektion 13 Kap 43Document19 pagesLektion 13 Kap 43Ritta AlshihabiNo ratings yet
- Nuclear PhysicsDocument46 pagesNuclear PhysicsAmit PaulNo ratings yet
- 22 - Nuclear EnergyDocument6 pages22 - Nuclear EnergyRonin SonNo ratings yet
- ENCHML130 1 Energy 5 Nuclear 1Document27 pagesENCHML130 1 Energy 5 Nuclear 1G7 SJ-01 Cabataña, MichailaNo ratings yet
- Nuclear Binding Energy: For The "Special" AP Physics Students Montwood High School R. CasaoDocument22 pagesNuclear Binding Energy: For The "Special" AP Physics Students Montwood High School R. CasaoAris JuliantoNo ratings yet
- Energy Conversion: Nuclear Power Plants: Nuclear Physics BasicsDocument22 pagesEnergy Conversion: Nuclear Power Plants: Nuclear Physics BasicsAhmed SamyNo ratings yet
- Nuclear Chemistry: Topic 3bDocument50 pagesNuclear Chemistry: Topic 3bJeam Russell AlfaroNo ratings yet
- Structure of Atom: Made By:dr. Isha Jaiswal Moderator: Dr. S.P.MishraDocument37 pagesStructure of Atom: Made By:dr. Isha Jaiswal Moderator: Dr. S.P.MishraRafael OrtegaNo ratings yet
- 13 NucleiDocument20 pages13 NucleipaperNo ratings yet
- ch21 Nuclear ChemDocument25 pagesch21 Nuclear ChemKeasNo ratings yet
- Chem11e Group 02 Nuclear ChemistryDocument36 pagesChem11e Group 02 Nuclear ChemistryArdent BautistaNo ratings yet
- Radioactive DecayDocument25 pagesRadioactive DecayAlex JosephNo ratings yet
- Lecture-1 - Atomic & Nuclear Physics - FundamentalsDocument17 pagesLecture-1 - Atomic & Nuclear Physics - Fundamentalstrr367267No ratings yet
- Chapter 11 Nuclear StructureDocument38 pagesChapter 11 Nuclear StructureAimi NabilaNo ratings yet
- Physics A Level - Nuclear-PhysicsDocument41 pagesPhysics A Level - Nuclear-PhysicsKeanan WongsoNo ratings yet
- Nuclear Chemistry: P. Nagaraja Assistant Professor in Chemistry Ap Iiit, RK Valley, RguktDocument27 pagesNuclear Chemistry: P. Nagaraja Assistant Professor in Chemistry Ap Iiit, RK Valley, RguktGORIPARTHI PENCHALA PRASADNo ratings yet
- 05 - Nuclear Energy Fission PDFDocument52 pages05 - Nuclear Energy Fission PDFusama riazNo ratings yet
- Nuclear Power Engineering: Aiub Dr. M. Tanseer Ali NPWR Lec 02 /1Document20 pagesNuclear Power Engineering: Aiub Dr. M. Tanseer Ali NPWR Lec 02 /1Naushed NihalNo ratings yet
- Atomic Theory & Structure: Oakland Schools Chemistry Resource UnitDocument52 pagesAtomic Theory & Structure: Oakland Schools Chemistry Resource UnithendraprimaNo ratings yet
- Lecture 36Document27 pagesLecture 36Jaya SharmaNo ratings yet
- Dayschool 01 - Unit 01Document8 pagesDayschool 01 - Unit 01Nethmi SenadheeraNo ratings yet
- 12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive DatingDocument8 pages12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive Datingshawnah jamesNo ratings yet
- Nuclear ChemistryDocument27 pagesNuclear ChemistryveluselvamaniNo ratings yet
- IsotopesDocument2 pagesIsotopeshanniisme3000No ratings yet
- RadioactivityDocument22 pagesRadioactivityChoudhry AbdullahNo ratings yet
- CP CS24 NuclearPhysicsDocument1 pageCP CS24 NuclearPhysicsSiti Ipah LatipahNo ratings yet
- Nuclei in One Shot - Class Notes - LEC-6 - PPT-6 - Physics - Nuclei - Vijeta Series - Kshitiz Kanik Sir - RAM NIWASDocument61 pagesNuclei in One Shot - Class Notes - LEC-6 - PPT-6 - Physics - Nuclei - Vijeta Series - Kshitiz Kanik Sir - RAM NIWASprajwal jhaNo ratings yet
- The Nucleus: SPH1104/6 2016 DR P. BaricholoDocument20 pagesThe Nucleus: SPH1104/6 2016 DR P. BaricholograceNo ratings yet
- Nuclei: - . - Types of Elements: Isotopes: IsobarsDocument12 pagesNuclei: - . - Types of Elements: Isotopes: IsobarsrgryjhgsdvrtNo ratings yet
- A-Level Edexcel ChemistryDocument22 pagesA-Level Edexcel ChemistryElyssa Mcpe100% (1)
- Nuclear ModelDocument11 pagesNuclear Modelzeamayf.biasNo ratings yet
- Atomic Structure - : ElectronDocument4 pagesAtomic Structure - : ElectronAinne Joy DimapilisNo ratings yet
- Atom Atom: Electron, The Proton and The NeutronDocument29 pagesAtom Atom: Electron, The Proton and The Neutronrehab ebraheemNo ratings yet
- Nuclear Physics: An OverviewDocument29 pagesNuclear Physics: An OverviewabdulbaseerNo ratings yet
- Mithun's Physics Revision Notes (Unit 1)Document6 pagesMithun's Physics Revision Notes (Unit 1)Mithun Dev SabaratnamNo ratings yet
- 02 Nuclear Chemistry R1Document60 pages02 Nuclear Chemistry R1April ImNo ratings yet
- Lect No 1 Nuclear Imaging FRCR PhysicsDocument22 pagesLect No 1 Nuclear Imaging FRCR Physicszlatan.srt8No ratings yet
- Nuclear Physics - PreMedDocument19 pagesNuclear Physics - PreMedNoor Ul ArfeenNo ratings yet
- Nuclei IDocument20 pagesNuclei IShoaibNo ratings yet
- Nuclei - Aakash RM Modules (@TEAMFLOOD)Document36 pagesNuclei - Aakash RM Modules (@TEAMFLOOD)sachinpoonia43825No ratings yet
- Nucleus and Its Characterstics: Prof - Arun Bharti Department of Physics University of JammuDocument41 pagesNucleus and Its Characterstics: Prof - Arun Bharti Department of Physics University of JammuSurbhi guptaNo ratings yet
- VIP Nuclear BasicsDocument166 pagesVIP Nuclear BasicsgetachewNo ratings yet
- Nuclear Chemistry 20-10-2020Document16 pagesNuclear Chemistry 20-10-2020Manohar MaripeNo ratings yet
- Unit 1Document23 pagesUnit 1mtayyab zahidNo ratings yet
- Fisika Inti Nuclear PhysicsDocument35 pagesFisika Inti Nuclear PhysicsRian Galileo MawonNo ratings yet
- Nuclear PDFDocument16 pagesNuclear PDFAlisha AkramNo ratings yet
- Chapter 21 - Modified by Rabeay 2022Document87 pagesChapter 21 - Modified by Rabeay 2022s-islam.safwatNo ratings yet
- 页面提取自-Chemistry for the IB Diploma Coursebook, 2nd EditionDocument1 page页面提取自-Chemistry for the IB Diploma Coursebook, 2nd EditionEshowbooks EbooksNo ratings yet
- 1.1 CchemDocument1 page1.1 CchemcallumNo ratings yet
- CH 15Document53 pagesCH 15Ronalson SiraitNo ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3No ratings yet
- Nuclear Fusion Enhanced by Negative Mass – A Proposed Method and Device – (Part 1): Nuclear Fusion Enhanced by Negative Mass, #1From EverandNuclear Fusion Enhanced by Negative Mass – A Proposed Method and Device – (Part 1): Nuclear Fusion Enhanced by Negative Mass, #1No ratings yet
- AP Physics I KinematicsDocument5 pagesAP Physics I Kinematicsmahsan abbasNo ratings yet
- Ap22 FRQ Physics C Mechanics Set 1Document17 pagesAp22 FRQ Physics C Mechanics Set 1mahsan abbasNo ratings yet
- FDocument1 pageFmahsan abbasNo ratings yet
- Vibrations and Waves: Additional Practice BDocument3 pagesVibrations and Waves: Additional Practice Bmahsan abbasNo ratings yet
- FDocument1 pageFmahsan abbasNo ratings yet
- 11ap 1Document7 pages11ap 1mahsan abbasNo ratings yet
- AP Physc M Dynamics Presentation 2018-10-23Document235 pagesAP Physc M Dynamics Presentation 2018-10-23mahsan abbasNo ratings yet
- APC DynamicsDocument8 pagesAPC Dynamicsmahsan abbasNo ratings yet
- 11ap T1 Sec BDocument3 pages11ap T1 Sec Bmahsan abbasNo ratings yet
- 1 Forces and Motion C2b Part II - Moments & Newtons 3rd: iGCSE Edexcel 1-9 - MR PowellDocument42 pages1 Forces and Motion C2b Part II - Moments & Newtons 3rd: iGCSE Edexcel 1-9 - MR Powellmahsan abbasNo ratings yet
- Ap C Imp NotesDocument3 pagesAp C Imp Notesmahsan abbasNo ratings yet
- Chapter 09Document96 pagesChapter 09mahsan abbasNo ratings yet
- 11 AP Chapter02Document49 pages11 AP Chapter02mahsan abbasNo ratings yet
- Preview: Objectives Refraction of Light The Law of Refraction Sample ProblemDocument52 pagesPreview: Objectives Refraction of Light The Law of Refraction Sample Problemmahsan abbasNo ratings yet
- 03 Transfer of HeatDocument34 pages03 Transfer of Heatmahsan abbasNo ratings yet
- Vectors and Two-Dimensional MotionDocument48 pagesVectors and Two-Dimensional Motionmahsan abbasNo ratings yet
- Rotational Equilibrium and Rotational DynamicsDocument49 pagesRotational Equilibrium and Rotational Dynamicsmahsan abbasNo ratings yet
- The Laws of MotionDocument44 pagesThe Laws of Motionmahsan abbasNo ratings yet
- 3 Waves - Part D Light: iGCSE Edexcel 1-9 - MR PowellDocument51 pages3 Waves - Part D Light: iGCSE Edexcel 1-9 - MR Powellmahsan abbasNo ratings yet
- Rotational Motion and The Law of GravityDocument47 pagesRotational Motion and The Law of Gravitymahsan abbasNo ratings yet
- 1 Forces and Motion - Units A: iGCSE Edexcel 1-9 - MR PowellDocument5 pages1 Forces and Motion - Units A: iGCSE Edexcel 1-9 - MR Powellmahsan abbasNo ratings yet
- Magnetism: Teacher Notes and Answers 19 MagnetismDocument3 pagesMagnetism: Teacher Notes and Answers 19 Magnetismmahsan abbas100% (1)
- SIRIUS IC10 Complete English 2017Document1,490 pagesSIRIUS IC10 Complete English 2017Laurentiu CatalinNo ratings yet
- Dynamic Pumps: and Its TypesDocument10 pagesDynamic Pumps: and Its Typeszain bajwaNo ratings yet
- Coke Drum Structure Safety During Shutdowns Maintenance Moloney ExxonMobil DCU New Delhi 2013 PDFDocument38 pagesCoke Drum Structure Safety During Shutdowns Maintenance Moloney ExxonMobil DCU New Delhi 2013 PDFtogentongNo ratings yet
- Kiln PDFDocument134 pagesKiln PDFpanji akbarNo ratings yet
- Tutorial 02 Ele 290 - Power Ac Circuits - QuestionDocument5 pagesTutorial 02 Ele 290 - Power Ac Circuits - QuestionAmiruddinMohktarNo ratings yet
- Modelling and Simulation For Automatic Irrigation System With PV Solar TrackingDocument4 pagesModelling and Simulation For Automatic Irrigation System With PV Solar TrackingSarlangaNo ratings yet
- 7 2022 - Gas Grid Training (Part 1) FluxysDocument41 pages7 2022 - Gas Grid Training (Part 1) FluxysJesús CastellanosNo ratings yet
- Effect of Drilling Fluid Filter CakeDocument5 pagesEffect of Drilling Fluid Filter CakeJosé Luis MoralesNo ratings yet
- OranurDocument4 pagesOranurtriesteNo ratings yet
- P31321 Central TriflexFullRange Unificado REV1Document2 pagesP31321 Central TriflexFullRange Unificado REV1INGWIRBONo ratings yet
- Penyelesaian Tutorial Problem 40.3 Ebook BL. Theraja NO 1 Dan 2Document13 pagesPenyelesaian Tutorial Problem 40.3 Ebook BL. Theraja NO 1 Dan 2Restu RivalniNo ratings yet
- ESFR Cold Storage Manual A4 UD March 2008Document111 pagesESFR Cold Storage Manual A4 UD March 2008jantonio1952No ratings yet
- The Pec Board ExamDocument6 pagesThe Pec Board ExamJonna MayNo ratings yet
- 6035 205 Sample QuestionsDocument3 pages6035 205 Sample QuestionsDavid DaveyNo ratings yet
- Fuel Consumption by EngineDocument2 pagesFuel Consumption by EngineRie ReiNo ratings yet
- Labram Ii H: Innovative Mixing SolutionsDocument2 pagesLabram Ii H: Innovative Mixing SolutionsSanjay GadeNo ratings yet
- Powermax65/85/105 SYNC: Cut Charts GuideDocument60 pagesPowermax65/85/105 SYNC: Cut Charts GuideSantiago MontenegroNo ratings yet
- C6.4 Engine Timing CalibrationsDocument4 pagesC6.4 Engine Timing CalibrationsKopyuk Kopyuk Kopyuk KopyukNo ratings yet
- OneTron Energy PVT LTDDocument2 pagesOneTron Energy PVT LTDGeorge TaoNo ratings yet
- Kestrel Static MixersDocument3 pagesKestrel Static MixersGozuengineer GozuNo ratings yet
- Coveral MTS 1584 PDFDocument2 pagesCoveral MTS 1584 PDFArchit Jhunjhunwala100% (1)
- Energy SecurityDocument33 pagesEnergy SecurityDhruv SharmaNo ratings yet
- Noise Reduction in Gas Turbines Fact SheetDocument12 pagesNoise Reduction in Gas Turbines Fact SheetOM GOYALNo ratings yet
- Governor DrawingDocument6 pagesGovernor DrawingPratikTiwariNo ratings yet
- Laser Welding and CuttingDocument7 pagesLaser Welding and CuttingeliiiiiiNo ratings yet
- LEDeG Trainees' Hostel, LehDocument23 pagesLEDeG Trainees' Hostel, LehVeinesh Pratik50% (2)