Professional Documents
Culture Documents
4.0 The Pituitary Gland
4.0 The Pituitary Gland
Uploaded by
Homeground entertainment0 ratings0% found this document useful (0 votes)
9 views68 pagesThis document provides an overview of the pituitary gland. It discusses that the pituitary gland lies at the base of the brain and is made up of the anterior, intermediate (which is rudimentary in humans), and posterior lobes. The anterior lobe secretes hormones that control other endocrine glands and tissues. These include growth hormone, TSH, ACTH, LH, FSH, and prolactin. The posterior lobe stores and releases oxytocin and vasopressin from hypothalamic neurons. The pituitary is regulated by factors from the hypothalamus and helps coordinate many other endocrine systems.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides an overview of the pituitary gland. It discusses that the pituitary gland lies at the base of the brain and is made up of the anterior, intermediate (which is rudimentary in humans), and posterior lobes. The anterior lobe secretes hormones that control other endocrine glands and tissues. These include growth hormone, TSH, ACTH, LH, FSH, and prolactin. The posterior lobe stores and releases oxytocin and vasopressin from hypothalamic neurons. The pituitary is regulated by factors from the hypothalamus and helps coordinate many other endocrine systems.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
9 views68 pages4.0 The Pituitary Gland
4.0 The Pituitary Gland
Uploaded by
Homeground entertainmentThis document provides an overview of the pituitary gland. It discusses that the pituitary gland lies at the base of the brain and is made up of the anterior, intermediate (which is rudimentary in humans), and posterior lobes. The anterior lobe secretes hormones that control other endocrine glands and tissues. These include growth hormone, TSH, ACTH, LH, FSH, and prolactin. The posterior lobe stores and releases oxytocin and vasopressin from hypothalamic neurons. The pituitary is regulated by factors from the hypothalamus and helps coordinate many other endocrine systems.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 68
PROF. N.
SYABBALO
MB., ChB., PhD., FCCP., FRS., FIBA
Professor of Physiology & Medicine
THE PITUITARY GLAND
INTRODUCTION
The pituitary gland, or hypophysis, lies in a pocket
of the sphenoid bone at the base of the brain
It is a coordinating center for control of many
downstream endocrine glands, some of which are
discussed in the subsequent chapters
In many ways, it can be considered to consist of at
least two (and in some species, three) separate
endocrine organs that contain a plethora of
hormonally active substances
THE PITUITARY GLAND
The anterior pituitary secretes thyroid-stimulating
hormone (TSH), adrenocorticotropic hormone
(ACTH), luteinizing hormone (LH), follicle-
stimulating hormone (FSH), prolactin, and
growth hormone
It receives almost its blood supply from the portal
hypophysial vessels that pass initially through the
median eminence, a structure immediately below the
hypothalamus
TABLE NS 1-1 Hormones secreted by
the anterior pituitary gland
Adrenocorticotropic hormone (corticotropin, ACTH)
Thyroid stimulating hormone (thyrotropin, TSH)
Growth hormone
Follicle- stimulating hormone (FSH)
Luteinizing hormone (LH)
Prolactin (PRL)
Polypeptide β-lipotropin (β-LPH)
THE PITUITARY GLAND
This vascular arrangement positions the cells of the
anterior pituitary to respond efficiently to regulatory
factors release from the hypothalamus
Of the listed hormones, prolactin acts on the breast
The remaining five are, at least in part, tropic hormones;
that is they stimulate secretion of hormonally active
substances by other endocrine glands or, in the case of
growth hormone, the liver and other tissues
Another example is TSH, which stimulates secretion of
the thyroid gland
THE PITUITARY GLAND
The tropic hormones for some endocrine glands are
discussed in the chapter on that gland
However, the gonadotropins FSH and LH, along with
prolactin, are covered here
The posterior pituitary in mammals consists
predominantly of nerves that have their cell bodies in
the hypothalamus (supraoptic and paraventricular and
nuclei)
It stores oxytocin and vasopressin in the terminals
of these neurons
THE PITUITARY GLAND
The hypothalamus and median eminence plays an
overall role in regulating both the anterior and
posterior pituitary hormones
In some species, there is also a well developed
intermediate lobe of the pituitary, whereas in
humans it is rudimentary
Nevertheless, the intermediate lobe, as well as the
anterior pituitary contain active derivatives of the
proopiomelanocortin (POMC) molecule that
regulate skin pigmentation, among other functions
THE PITUITARY GLAND
This chapter will focus predominantly on growth
hormone and its role in growth and in facilitating the
activity of other hormones, along with a number of
general consideration about the pituitary
The melanocyte-stimulating hormones (MSH) of the
intermediate lobe of the pituitary, α-MSH and β-
MSH,will also be touched upon
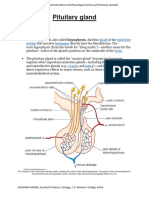
MORPHOLOGY
GROSS ANATOMY
The anatomy of the pituitary gland is summarized in
Figure 18-1 and discussed in detail in Chapter 17
The posterior pituitary is made up largely of the
endings of axons from the supraoptic and
paraventricular nuclei of the hypothalamus and arises
initially as an extension of this structure
The anterior pituitary, on the other hand, contains
endocrine cells that store its characteristic hormones,
and arises embryologically from an invagination of the
pharynx (Rathke’s pouch)
MORPHOLOGY
GROSS ANATOMY
In species where it is well developed, the intermediate
lobe is formed in the embryo from the dorsal half of
Rathke’s pouch, but is closely adherent to the posterior
lobe in the adult
It is separated from the anterior lobe by the remains of
the cavity in Rathke’s pouch, the residual cleft
HISTOLOGY
In the posterior lobe, the endings of the supraoptic
and paraventricular axons can be observed in close
relation to blood vessels
Pituicytes, stellate cells that are modified astrocytes
are also present
The intermediate lode is rudimentary in humans and
a few other mammal species
In these species, most of its cells are incorporated in
the anterior lobe
HISTOLOGY
Along the residual cleft are small thyroid-like follicles, some
containing a little colloid
The function of the colloid, if any, is unknown
The anterior pituitary is made up of interlacing cell cords
and an extensive network of sinusoidal capillaries
The endothelium of the capillaries is fenestrated, like that in
other endocrine organs
The cells contain granules of stored hormones that are
extruded from the cell by exocytosis
Their constituents then enter the capillaries to be conveyed
to target tissues or endocrine glands
CELL TYPES IN THE ANTERIOR
PITUITARY
Five types of secretory cells have been identified in the
anterior pituitary by immunocytochemistry and electron
microscopy
The cell types are the somatotropes, which secrete growth
hormone
The lactotropes (also called mammotropes), which secrete
prolactin
The corticotropes, which secrete ACTH
The thyrotropes, which secrete TSH; and gonadotropes,
which secrete FSH and LH
The characteristics of these cells are summarized in Table 18-1
TABLE 18-1 Hormone-secreting cells of
the human anterior pituitary gland
Cell type Hormone % of Total cells
Somatotrope GH 50
Lactotrope Prolactin 10-30
Corticotrope ACTH 10
Thyrotrope TSH 5
Gonadotrope FSH, LH 20
Percentage of total secretory cells (%)
CELL TYPES IN THE ANTERIOR
PITUITARY
Some cells may contain two or more hormones, for
example, gonadotropes which contain both FSH and
LH in their granules
It is also notable that the three pituitary glycoproteins
hormones, FSH, LH, and TSH, while being made up of
two subunits, all share a common α-subunit that is the
product of a single gene
It has the same amino acid composition in each
hormone, although their carbohydrate residue vary
CELL TYPES IN THE ANTERIOR
PITUITARY
The α-subunit must be combined with a β-subunit
characteristic of each hormone for maximal
physiologic activity
The β-subunit, which are produced by separate genes
and differ in structure, confer hormonal specificity
The α-subunit, are remarkably interchangeable and
hybrid molecules can be created
In addition, the placental glycoprotein gonadotropin
human chorionic gonadotropin (hCG) has α and β
subunits
CELL TYPES IN THE ANTERIOR
PITUITARY
The anterior pituitary also contains folliculostellate cells that
send processes between the granulated secretory cells
These cells produce paracrine factors that regulate growth and
function of secretory cells
Indeed, the anterior pituitary can adjust the relative proportion
of secretory cell type to meet varying requirements for different
hormones at different life stages
This plasticity has recently been ascribed to the presence of a
small number of pluripotent stem cells that persist in the adult
gland
These pluripotent cells may different into different secretory cells
PROOPIOMELANOCORTIN AND
DERIVATIVES
BIOSYNTHESIS
Intermediate-like cells, if present, and corticotropes of
the anterior lobe both synthesize a large precursor
protein that is cleaved to form a family of hormones
After the removal of the signal peptide, this
prohormone is known as proopiomelanocortin
(POMC)
This molecule is also synthesized in the
hypothalamus, the lungs, the gastrointestinal tract,
and the placenta
PROOPIOMELANOCORTIN AND DERIVATIVES
The structure of POMC, as well as its derivatives is
shown in Figure 18-2
In corticotropes, it is hydrolyzed to ACTH and β-
lipotropin (LPH), plus a small amount of β-
endorphin, and these substances are secreted
In the intermediate lobe cells, POMC is hydrolyzed to
corticotropin-like intermediate-lobe peptide (CLIP), γ-
LPH, and appreciable quantities of β-endorphin
PROOPIOMELANOCORTIN AND
DERIVATIVES
The functions, if any, of CLIP and γ-LPH are unknown
whereas β-endorphin is an opioid peptide that has the
five amino acid residues of met-enkephalin at its
amino terminal end
The melanotropins α and β-MSH are also formed
However, the intermediate lobe in humans is
rudimentary, and it appears that neither α-MSH nor β-
MSH is secreted in adults
In some species, however, the melanotropins have
important physiological functions
CONTROL OF SKINCOLORATION
AND PIGMENT ABNORMALITIES
Fish, reptiles, and amphibia change the color of their
skin for thermoregulation, camouflage, and behavioral
displays
They do this in part by moving black or brown
granules into or out of the periphery of pigmented
cells called melanophores
The granules are made up of melanins, which are
synthesized from dopamine and dopaquinone
CONTROL OF SKIN COLORATION
AND PIGMENT ABNORMALITIES
The movement of these granules is controlled by a
variety of hormones and neurotransmitters, including
α- and β-MSH, melanin-concentrating hormone,
melatonin, and catecholamine
Mammals have no melanophores containing pigment
granules that disperse and aggregate, but they do have
melanocytes, which have multiple processes
containing melanin granules
Melanocytes express melanotropin-1 receptors
CONTROL OF SKIN COLORATION
AND PIGMENT ABNORMALITIES
Treatment with melanotropins accelerates melanin
synthesis, causing readily detectable darkening of the skin
in humans in 24 h
As noted above, α- and β-MSH do not circulate in adult
humans, and their function is unknown
However, ACTH binds to melanotropin-1 receptors
Indeed, the pigmentary changes in several human
endocrine diseases are due to changes in circulating ACTH
For example, abnormal pallor is a hallmark of
hypopituitarism
CONTROL OF SKIN COLORATION
AND PIGMENT ABNORMALITIES
Hyperpigmentation occurs sometimes in patients with
Cushing’s syndrome, Addison’s disease, and Nelson’s
disease due to primary adrenal disease
In Cushing disease pigmentation occur most often
following administration of synthetic steroids or
ACTH
In Addison’ s disease there is destruction of the entire
adrenal cortex
Reduced cortisol levels lead, through feedback, to
increased CRH and ACTH production
CONTROL OF SKINCOLORATION
AND PIGMENT ABNORMALITIES
Indeed, the presence of hyperpigmentation in association with
adrenal insufficiency rules out the possibility that the
insufficiency is secondary to pituitary or hypothalamic disease
because in these conditions plasma ACTH is not increased
Other disorders of pigmentation result from peripheral
mechanisms or genetic mechanisms and include oculocutaneous
albinism and vitiligo
Oculocutaneous albinism
Oculocutaneous albinos result from a range of genetic
abnormalities that lead to reduced melanin biosynthesis in the
skin, hair and eyes; or a congenital inability to synthesize melanin
CONTROL OF SKIN COLORATION
AND PIGMENT ABNORMALITIES
The number of melanocytes is normal (in contrast to vitiligo)
This can result from a variety of different genetic defects in the
pathways for melanin synthesis
Albinism is usually inherited as an autosomal recessive trait and
there are several different types
Type 1 albinism is due to a defect in tyrosinase gene, whose
product is rate-limiting in the production of melanin
Affected individuals have an almost complete absence of pigment
in the skin and hair at birth, and failure of melanin production in
the iris and retina
Patients have photophobia, poor vision, and rotational
nystagmus
CONTROL OF SKIN COLORATION
AND PIGMENT ABNORMALITIES
A second type is due to a defect in the P gene, which
encodes an ion channel protein in the melanosome
Patients may have gross reduction of melanin in the
skin and eyes, but are more mildly affected than type 1
albinos
Oculocutaneous albinos are at grossly increased risk
of sunburn and skin cancer
In equatorial regions, many die from squamous cell
carcinoma (SCC) or, more rarely melanoma in early
adult life
CONTROL OF SKIN COLORATION
AND PIGMENT ABNORMALITIES
Piebaldism is characterized by patches of skin that lack
melanin as a result of congenital defects in the migration
of the pigment cell precursors from the neural crest
during embryonic development
Not only the condition but also the precise pattern of the
loss is passed from one generation to the next
Vitiligo is an acquired condition affecting 1% of the
population worldwide
It involves a similar patchy loss of melanin, but the loss
develop progressively after birth secondary to an
autoimmune process that target the melanocytes
CONTROL OF SKIN COLORATION
AND PIGMENT ABNORMALITIES
This type of disease is associated with other autoimmune disease
A positive family history of vitiligo or other organ-specific
autoimmune disease is relatively common in those with the
disease
Generalized vitiligo is often symmetrical and frequently involves
face, hands, wrist, knees, neck, genitalia, and around body
orifices
The hair of the scalp, beard, eyebrows and lashes may also
depigment
Sensation in the depigmented patches is normal (unlike in
tuberculoid leprosy)
GROWTH HORMONE
BIOSYNTHESIS AND CHEMISTRY
The long arm of human chromosome 17 contains the
growth hormone-hCS cluster that contains five genes;
one, hGH-N codes for the most abundant (“normal”)
form of growth hormone
A second, hGH-V, codes for the variant form of growth
hormone
Two code for human chorionic somatomammatropin
(hCS); and the fifth is probably an hCS peudogene
GROWTH HORMONE
BIOSYNTHESIS AND CHEMISTRY
Growth hormone that is secreted into the circulation
by the pituitary gland consists of a complex mixture of
hGH-N, peptides derived from this molecule with
varying degrees of posttranslational modifications,
such as glycosylation, and a splice variant of hGH-N
that lacks amino acids 32-46
The physiologic significance of this complex array of
hormones has yet to be fully understood
Particularly since their structural similarities make it
difficult to assay for each species separately
GROWTH HORMONE
BIOSYNTHESIS AND CHEMISTRY
Nevertheless, there is emerging evidence that, while
the various peptides share a broad range of functions,
they may occasionally exert actions in opposition to
one another
hGH-V and hCS, on the other hand, are primarily
products of the placenta, and as a consequence are
only found in appreciable quantities in the circulation
during pregnancy (see Chapter 22)
SPECIES SPECIFICITY
The structure of growth hormone varies considerably
from one species to another
Porcine and simian growth hormones have only a
transient effect in the guinea pig
In monkeys and humans, bovine and porcine growth
hormones do not even have a transient effect on
growth, although monkey and human growth
hormone are fully active in both monkeys and humans
SPECIES SPECIFICITY
These fact are relevant to public health discussions
surrounding the presence of bovine growth hormones
(used to increase milk production) in dairy products
As well as the popularity of growth hormone
supplements, marketed via the internet, with body
builders
Controversially, recombinant human growth hormone
has also been given in children who are short in
stature, but otherwise healthy (ie, without growth
hormone deficiency), with apparently limited results
PLASMA LEVELS, BINDING, AND
METABOLISM
A portion of circulating growth hormone is bound to a
plasma protein that is a large fragment of the
extracellular domain of the growth hormone receptor
It appears to be produced by cleavage of receptors in
humans, and its concentration is an index of the
number of growth hormone receptors in the tissues
Approximately 50% of the circulating pool of growth
hormone activity is in the bound form, providing a
reservoir of the hormone to compensate for the wide
fluctuation that occur in secretion
PLASMA LEVELS, BINDING, AND
METABOLISM
The basal plasma growth hormone level measured by
radioimmunoassay in adult humans is normally less
than 3 ng/ml
This represents both the protein-bound and the free
form
Growth hormone is metabolized rapidly, at least in
part in the liver
The half-life of circulating growth hormone in
humans is 6-20 min, and the daily growth hormone
output has been calculated to be 0.2-1.0 mg/d in adults
GROWTH HORMONE RECEPTORS
The growth hormone receptor is a 620-amino-acid
protein with a large extracellular portion, a
transmembrane domain, and a large cytoplasmic
portion
It is a member of the cytokine receptor superfamily
Growth hormone has two domains that can bind to its
receptor, and when it binds one receptor, the second
binding site attracts another, producing a homodimer
Dimerization is essential for receptor activation which
leads to several principal signaling pathways
GROWTH HORMONE RECEPTORS
Growth hormone has widespread effects in the body,
so even though it is not yet possible precisely to
correlate intracellular and whole body effects
It is also not surprising that, like insulin, growth
hormone activates many different intracellular
signaling cascades
Of particular note is its activation of the JAK2-STAT
pathway
JAK2 is a member of the Janus family of cytoplasmic
tyrosine kinases
GROWTH HORMONE RECEPTORS
STATs (for signal transducers and activators of
transcription) are a family of cytoplasmic transcription
factors that, upon phosphorylation by JAK kinases,
migrate to the nucleus where they activate various
genes
JAK-STAT pathways are known also to mediate the
effect of prolactin and other growth factors
EFFECT ON GROWTH
In young animals in which the epiphyses have not yet
fused to the long bones, growth is inhibited by
hypophysectomy and stimulated by growth hormone
Chondrogenesis is accelerated, and the cartilaginous
epiphysial plate widen, they lay down more bone
matrix at the end of the long bones
In this way, stature is increased
Prolonged treatment of young animals with growth
hormone leads to gigantism
EFFECT ON GROWTH
When the epiphyses are closed, linear growth is no
longer possible
In this case, an overabundance of growth hormone
produces the pattern of bone and soft tissue
deformation known to humans as acromegaly
The size of most of the viscera are increased
The protein content of the body is increased, and the
fat content is decreased (see Clininal Box 18-1)
EFFECTS ON PROTEIN & ELECTROLYTE
HOMEOSTASIS
Growth hormone is a protein anabolic hormone and
produces a positive nitrogen and phosphorus balance,
a rise in plasma phosphorus, and a fall in blood urea
nitrogen and amino acid levels
In adults with growth hormone deficiency,
recombinant growth hormone produces an increase in
lean body mass and a decrease in body fat, along with
an increase in metabolic rate and a fall in plasma
cholesterol
EFFECTS ON PROTEIN & ELECTROLYTE
HOMEOSTASIS
Gastrointestinal absorption of Ca is increased, Na and
K excretion is reduced by an action independent of the
adrenal glands, probably because these electrolytes are
diverted from the kidneys to the growing tissues
On the other hand, excretion of amino 4-
hydroxyproline is increased during the growth,
reflective of the ability of growth hormone to facilitate
the synthesis of soluble collagen
EFFECT ON CARBOHYDRATE & FAT
METABOLISM
The action of growth hormone on carbohydrate
metabolism are discussed in Chapter 24
At least some form of growth hormone are diabetogenic
because they increase hepatic glucose output and exert
an anti-insulin effect on muscle
Growth hormone is also ketogenic and increases
circulating free fatty acids (FFS) levels
The increase in FFA, which takes several hours to
develop, provide a ready source of energy for the tissues
during hypoglycaemia, fasting, and stressful stimuli
EFFECT ON CARBOHYDRATE & FAT
METABOLISM
Growth hormone does not stimulate β cells of the
pancreas directly, but it increases the ability of the
pancreas to respond to insulinogenic stimuli such as
arginine and glucose
This is an additional way growth hormone promotes
growth, since insulin has a protein anabolic effect
TABLE 18-5 DIRECT AND INDIRECT ACTIONS
OF GROWTH HORMONE AND IGF1
Growth hormone
Epiphyseal growth
Cartilage growth
Protein synthesis
Lipolysis
Decreased insulin sensitivity
Na retention
IGF1
Epiphyseal growth
Protein synthesis
Antilipolytic activity
Insulin-like activity
SOMATOMEDINS
The effects of growth hormone on growth, cartilage,
and protein metabolism depend on an interaction
between growth hormone and somatomedins
Somatomedins are polypeptide growth factors
secreted by the liver and other tissues
The first of these factors isolated was called sulfation
factor because it stimulated the incorporation of
sulfate into cartilage
However, it also stimulates collagen formation, and its
name was changed to somatomedin
SOMATOMEDINS
It then became clear that there are a variety of different
somatomedins and that they are members of an
increasingly large family of growth factors that affect
many different tissues and organs
The principal (and in humans probably the only)
circulating somatomedins are insulin-like growth
factor I (IGF-I, somatomedin C), and IGF II
These factors are closely related to insulin, except that
their C chains are not separated and they have an
extension of the A chain called the D domain (Figure
18.4)
SOMATOMEDINS
The hormone relaxin is also a member of this family
Humans have two related relaxin isoforms, and both
resemble IGF-II
In humans a variant form of IGF-1 lacking three amino
terminals amino acid residues has been found in the
brain, and there are several variant forms of human
IGF-II (Figure 18-4)
SOMATOMEDINS
The mRNAs for IGF-I and IGF-II are found in the liver, in
cartilage, and in many other tissues, indicating that they
are likely synthesized in these tissues
The properties of IGF-I, IGF-II, and insulin are compared
in Table 18-2
Both IGF-I and IGF-II are tightly bound to proteins in
plasma, and, at least for IGF-I, this prolongs their half-life
in the circulation
Six different IGF-binding proteins, with different patterns
of distribution in various tissues, have been identified
SOMATOMEDINS
All are present in plasma, with IGF-binging protein-3
(IGFBP-3) accounting for 95% of the binding in the
circulation
The contribution of the IGFs to the insulin-like activity in
blood is discussed in Chapter 24
The IGF-I receptor is very similar to the insulin receptor
and probably uses similar or identical intracellular
signaling pathways
The IGF-II receptor has a distinct structure and is involved
in intracellular targeting of acid hydrolases and other
proteins to intracellular organelles
SOMATOMEDINS
Secretion of IGF-I is independent of growth hormone
before birth but it is stimulated by growth hormone
after birth, and it has pronounced growth-stimulating
activity
Its concentration in plasma rises during childhood
and peaks at the time of puberty, then declines to low
levels in old age
IGF-II is largely independent of growth hormone and
plays a role in the growth of the fetus before birth
SOMATOMEDINS
In human fetuses in which it is overexpressed, several
organs, especially the tongue, other muscles, kidneys,
heart, and liver, develop out of proportion to the rest
of the body
In adults, the gene for IGF-II is expressed only in the
choroid plexus and meninges
TABLE 18-2 Comparison of insulin
and the insulin-like growth factors
Insulin IGF-I
Other name … Somatomedin C
No. of amino acids 51 70
Source Pancreas Liver, other tissues
Regulation Glucose Growth hormone
Plasma level 0.3-2 ng/ml 10-700 ng/ml
Plasma BP No Yes
Physiologic role Metabolism Skeletal and cartilage growth
IGF-I peaks at puberty; BP, binding proteins
TABLE 18-2 Comparison of insulin
and the insulin-like growth factors
Insulin IGF-II
Other names … MSA
No. of amino acids 51 67
Source Pancreas Diverse
Regulation Glucose Unknown
Plasma level 0.3-2 ng/ml 300-800 ng/ Plasma BP No Yes
Physiologic role Metabolism Growth during fetal
development
MSA, Multiplication stimulating activity
CLINICAL BOX 18-1
GIGANTISM & ACROMEGALY
Tumors of the somatotropes of the anterior pituitary
(pituitary adenomas or somatotroph adenomas)
secrete large amounts of growth hormone, leading to
gigantism in children (if acquired before epiphyseal
fusion) and acromegaly in adults
If the tumor arises before puberty, the individual may
grow to an extraordinary height
After linear growth is no longer possible, on the other
hand, the characteristic features of acromegaly arise
CLINICAL BOX 18-1
GIGANTISM & ACROMEGALY
These include changes in appearance, enlargement of
the hands and feet, vertebral changes attributable to
osteoarthritis, hirsutism, and protruding of the
supraorbital ridges and lower jaw (prognathism)
Enlargement of lips, nose and tongue
Cardiomyopathy, hypertension and enlargement of
the liver
Sweating, headache, soft tissue swelling and sleep
apnoeas are particular useful features of persistent GH
secretion
CLINICAL BOX 18-1
GIGANTISM & ACROMEGALY
Headache is very common in acromegaly and may be
severe even with small tumours
It is often improved after surgical cure or with
somatostatin analogues
In the remainder the diagnosis is made by an alert
observer in another clinic, eg, GP, diabetic, hypertension,
dermatology, dental
Abnormal growth of the internal organs, eg
cardiomyopathy and liver may eventually impair their
function such that the condition, which has an insidious
onset, can prove fatal if left untreated
TABLE NS 18-1 Symptoms and
signs of acromegaly
Change in appearance
Increased size of hands/feet
Headache
Excessive sweating
Visual disturbance
Sleep apnoea
Amenorrhoea or oligomenorrhoea
Galactorrhoea
Impotence or loss of libido
TABLE NS 18-1 Symptoms and
signs of acromegaly
Deep voice
Breathlessness
Polyuria/polydipsia
Pain/tingling in hands
Joint pains
Muscular weakness
CLINICAL BOX 18-1
GIGANTISM & ACROMEGALY
The overall incidence of gigantism is approximately 3-4
million per year and the prevalence is 50-80/million world-
wide
Acromegaly usually occurs sporadically although gene
mutations can rarely give rise to familial acromegaly,
typically the AIP gene in familial isolated pituitary
adenoma
One-third of patients present with change in appearance,
one-quarter with visual field defects or headache
Sleep apnoea is common and requires investigation and
treatment
CLINICAL BOX 18-1
GIGANTISM & ACROMEGALY
Hypersecretion of growth hormone is accompanied by
hypersecretion of prolactin in 20-40% of patients with
acromegaly
Approximately 4% of the patients with acromegaly
develop lactation in the absence of pregnancy
About 25% of patients have impaired glucose tolerance
tests, and 10% have type 2 diabetes mellitus
Acromegaly can be caused by extra-pituitary as well as
intrapituitary growth hormone secreting tumors and by
hypothalamic tumors that secrete GRH, but the latter are
rare
THERAPEUTIC HIGHLIGHTS
Untreated acromegaly result in markedly reduced survival
Most death occur from heart failure, coronary artery
disease, and hypertension-related causes
In addition, there is an increase in death due to neoplasia,
particularly colonic cancer (2-3 times)
Therefore treatment is indicated in all except the elderly
or those with minimal abnormalities
The mainstay of therapy remains the use of somatostatin
analogues that inhibit the secretion of growth hormone
THERAPEUTIC HIGHLIGHTS
Octreotide and lanreotide are synthetic analogues of
somatostatin that selectively act on somatostatin
receptor subtypes (SST2 and SST5), which are highly
expressed in growth-hormone secreting tumors
They reduce GH and IGF levels in most patients but not
all achieve treatment target
Dopamine agonists
Dopamine agonists act on D2 receptors and can be
given to shrink tumors prior to definite therapy or to
control symptoms and persisting GH secretion
THERAPEUTIC HIGHLIGHTS
They are probably most effective in mixed growth-
hormone producing (somatotroph) and prolactin-
producing (mammotroph) tumors
Typical doses are bromocriptine 10-60 mg daily or
cabergoline 0.5 mg daily (higher than for
prolactinoma)
A growth hormone receptor antagonist has recently
become available and has been found to reduce plasma
IGF-I and produce a clinical improvement in cases of
acromegaly that fail to respond to other treatments
THERAPEUTIC HIGHLIGHTS
Pegvisomant (a genetically modified analogue of GH)
is a GH receptor antagonist which has its effect by
binding to and preventing deminerization of the GH
receptor
It does not lower GH levels or reduce tumor size but has
been shown to normalize IGF-I levels in 90% of patients
It is given by a daily injection and its main role at
present is treatment of patients in whom GH and IGF
levels cannot be reduced to safe levels with somatostatin
analogues alone
THERAPEUTIC HIGHLIGHTS
Surgical removal of the pituitary tumor is helpful in both
acromegaly and gigantism, but sometimes challenging to
perform due to the tumor’s often invasive nature
Trans-sphenoidal surgery is the appropriate first-line
therapy
It will result in clinical remission in a majority of cases
(60-90%) with pituitary microadenomas but only 50% of
those with macroadenomas
In any case, adjuvant pharmacological therapy must often
be continued after surgery to control ongoing symptoms
THERAPEUTIC HIGHLIGHTS
Pituitary radiotherapy
External radiotherapy is normally used after pituitary
surgery fails to normalize GH levels rather than a
primary therapy
It is usually combined with a somatostatin analogue,
dopamine agonist or GH antagonist because of the
slow biochemical response to radiotherapy
The response may take 10 years or more, and is then
associated with hypopituitarism which makes it un
attractive in patients of reproductive age
You might also like
- Main Dip Step 2ck Notes Info Doc - Read OnlyDocument305 pagesMain Dip Step 2ck Notes Info Doc - Read Only[161]Shuaib AktherNo ratings yet
- Dentin Book NBDE Part-2, 2016 PDFDocument1,512 pagesDentin Book NBDE Part-2, 2016 PDFAbdurahman Pathan100% (9)
- Understanding Muhammad Is A Psycho Biography by Ali SinaDocument5 pagesUnderstanding Muhammad Is A Psycho Biography by Ali Sinaviviansteven75% (4)
- IP-Worksheet-3-Endocrine System The Hypothalamic-Pituitary Axis (Estomagulang)Document2 pagesIP-Worksheet-3-Endocrine System The Hypothalamic-Pituitary Axis (Estomagulang)Filmae Estomagulang0% (1)
- Carte de Abstracte Cimsc 2017 1Document135 pagesCarte de Abstracte Cimsc 2017 1Remus BobârnacNo ratings yet
- Pituitary GlandDocument22 pagesPituitary GlandSara Musavi100% (2)
- Hormones of PituitaryDocument35 pagesHormones of Pituitarybhagirath kansara100% (1)
- Hypothalamus - Anterior Pituitary and Their HormonesDocument138 pagesHypothalamus - Anterior Pituitary and Their HormonesML Rodriguez100% (1)
- Pitutary GlandDocument3 pagesPitutary GlandMariannNo ratings yet
- Pituitary Gland - Structure and Function by Dr. Shushma KumariDocument6 pagesPituitary Gland - Structure and Function by Dr. Shushma Kumariakshdeep1747No ratings yet
- ImmunologyDocument2 pagesImmunologyDebapriya HazraNo ratings yet
- Endocrine SystemDocument14 pagesEndocrine SystemEdsel Ian S. FuentesNo ratings yet
- Pituitary Gland (Hypophysis)Document28 pagesPituitary Gland (Hypophysis)Nastase Daniela EcaterinaNo ratings yet
- Hypothalamus - Hypophysis Functional AnatomyDocument11 pagesHypothalamus - Hypophysis Functional AnatomyRajesh ReddyNo ratings yet
- The Endocrine System 3 PDFDocument47 pagesThe Endocrine System 3 PDFRifi OktasiaNo ratings yet
- The Endocrine SystemDocument101 pagesThe Endocrine SystemLeônidas ZazelisNo ratings yet
- Histology Lecture 7 - Endocrine SystemDocument20 pagesHistology Lecture 7 - Endocrine SystemMicahNo ratings yet
- Pituitary Hormones and Their Control by The HypothalamusDocument20 pagesPituitary Hormones and Their Control by The HypothalamusMustafa KadhemNo ratings yet
- Pituitary Hormones and Their Control by The HypothalamusDocument26 pagesPituitary Hormones and Their Control by The HypothalamusDr. AliNo ratings yet
- Histology of Endocrine SystemMKDocument54 pagesHistology of Endocrine SystemMKDo Ra EmonNo ratings yet
- Mhs-1Pituitary Gland PresentationDocument34 pagesMhs-1Pituitary Gland PresentationAyi Abdul BasithNo ratings yet
- Endocrinal PathologyDocument81 pagesEndocrinal PathologyshehlaNo ratings yet
- Endocrine System Group 9Document77 pagesEndocrine System Group 9Jei SanNo ratings yet
- Endocrine - HistoworldDocument12 pagesEndocrine - HistoworldKarl Torres Uganiza RmtNo ratings yet
- Physio RespiDocument17 pagesPhysio RespiJenica SorianoNo ratings yet
- Unit2-Hypothalamus and PituitaryDocument80 pagesUnit2-Hypothalamus and Pituitarypranutan739No ratings yet
- Histology Lec-11 EndocrineDocument10 pagesHistology Lec-11 EndocrineKevin C. AguilarNo ratings yet
- Lecture 2 - Pituitary Hormones and Their Control by The HypothalamusDocument39 pagesLecture 2 - Pituitary Hormones and Their Control by The HypothalamusHomeground entertainmentNo ratings yet
- Endokrin MW DitransletDocument7 pagesEndokrin MW DitransletSanta SaintNo ratings yet
- (HISTOLOGY) Endocrine SystemDocument11 pages(HISTOLOGY) Endocrine Systemwipi112No ratings yet
- Hypothalmo-Hypophysial Axis: by Dr. Luna PhukanDocument35 pagesHypothalmo-Hypophysial Axis: by Dr. Luna PhukanAmmar QuasmiNo ratings yet
- Capitulo Eje PDFDocument15 pagesCapitulo Eje PDFMilena ArdilaNo ratings yet
- English Sistem EndocrineDocument9 pagesEnglish Sistem EndocrineNurlaili YaniNo ratings yet
- Endocrine ResonanceDocument51 pagesEndocrine ResonanceEkta ManglaniNo ratings yet
- The Hypothalamus and Pituitary Gland: General Principles of Endocrine DiagnosisDocument13 pagesThe Hypothalamus and Pituitary Gland: General Principles of Endocrine DiagnosisKiprono Keitany TimothyNo ratings yet
- Histology of Endocrine SystemMKDocument54 pagesHistology of Endocrine SystemMKDo Ra EmonNo ratings yet
- Endocrine System Part 1.Document57 pagesEndocrine System Part 1.Jonathan Young XDNo ratings yet
- Biochemistry of Hormones (Part 1)Document31 pagesBiochemistry of Hormones (Part 1)arun231187100% (1)
- Endocrinology:: By: DR Suyash SharmaDocument186 pagesEndocrinology:: By: DR Suyash SharmaaalipuriaNo ratings yet
- BallsDocument91 pagesBallsJoseph RaofNo ratings yet
- Endocrine SystemDocument31 pagesEndocrine SystemPreeti ChouhanNo ratings yet
- ENDODocument18 pagesENDOJoezer Gumangan VeranoNo ratings yet
- Unit IXDocument10 pagesUnit IXPreeti ChouhanNo ratings yet
- Endocrinology Notes: Veterinary PhysiologyDocument16 pagesEndocrinology Notes: Veterinary PhysiologyBrian AllanNo ratings yet
- Endocrine System (Vertebrate) : HomeostasisDocument7 pagesEndocrine System (Vertebrate) : HomeostasisKaleem UllahNo ratings yet
- Endocrine System OutlineDocument17 pagesEndocrine System OutlineEsthermae PanadenNo ratings yet
- Overview of Endo Physiology - HandoutDocument19 pagesOverview of Endo Physiology - HandoutVDiesel99No ratings yet
- Chapter 8 The Endocrine SystemDocument56 pagesChapter 8 The Endocrine SystemSainuddinSaddinNo ratings yet
- New Microsoft Word DocumentDocument13 pagesNew Microsoft Word DocumentdinaNo ratings yet
- Human Anatomy Physiology Chapter 14 Endocrine System NotesDocument13 pagesHuman Anatomy Physiology Chapter 14 Endocrine System Notesadarshjain816No ratings yet
- Endocrine System: Mechanisms of Hormone ActionDocument13 pagesEndocrine System: Mechanisms of Hormone ActionSagun LamaNo ratings yet
- Pituitary GlandDocument15 pagesPituitary GlandPutri Agustin Mere100% (1)
- Hormone NotesDocument2 pagesHormone Notesapi-188978784100% (1)
- Chemical Control and CoordinationDocument19 pagesChemical Control and CoordinationShubhamNo ratings yet
- Endocrine System Anatomy and PhysiologyDocument17 pagesEndocrine System Anatomy and PhysiologyKBD0% (1)
- Functions of The Endocrine System: EquilibriumDocument20 pagesFunctions of The Endocrine System: EquilibriumKumah WisdomNo ratings yet
- Unit 2 Part 1 Patho 2 Kmu IhsanDocument33 pagesUnit 2 Part 1 Patho 2 Kmu IhsanRehan Khan RkNo ratings yet
- Hormones:: Signaling MoleculesDocument20 pagesHormones:: Signaling MoleculesSangeeta DwivediNo ratings yet
- Hypothalamus and Pituitary GlandDocument39 pagesHypothalamus and Pituitary GlandGish KioiNo ratings yet
- Endocrine SystemDocument52 pagesEndocrine SystemDishani DeyNo ratings yet
- Endocrine System Sem2Document14 pagesEndocrine System Sem2chandraboli majumdarNo ratings yet
- Chap 3 Reproductive Hormones of Female AnimalsDocument80 pagesChap 3 Reproductive Hormones of Female Animalsyabsiradaniel930No ratings yet
- The Pituitary GlandDocument3 pagesThe Pituitary Glandshrabasti89No ratings yet
- Pituitary Gland, Functions, Diseases, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandPituitary Gland, Functions, Diseases, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsRating: 3 out of 5 stars3/5 (1)
- Breech PresentationDocument27 pagesBreech PresentationHomeground entertainmentNo ratings yet
- Cervical CancerDocument87 pagesCervical CancerHomeground entertainmentNo ratings yet
- Cesarean SectionDocument36 pagesCesarean SectionHomeground entertainment100% (1)
- CaesareanDocument32 pagesCaesareanHomeground entertainmentNo ratings yet
- Proteins: Eric Mbindo NjunjuDocument76 pagesProteins: Eric Mbindo NjunjuHomeground entertainmentNo ratings yet
- Perinatal Asphyxia: Presenters: Patrick Peter BwalyaDocument37 pagesPerinatal Asphyxia: Presenters: Patrick Peter BwalyaHomeground entertainmentNo ratings yet
- Convulsive Disorders: Presenters: Kabwe Chanda EliasDocument30 pagesConvulsive Disorders: Presenters: Kabwe Chanda EliasHomeground entertainmentNo ratings yet
- Mutations: Eric Mbindo NjunjuDocument62 pagesMutations: Eric Mbindo NjunjuHomeground entertainmentNo ratings yet
- Biochemistry MBS 230: Eric Mbindo NjunjuDocument20 pagesBiochemistry MBS 230: Eric Mbindo NjunjuHomeground entertainmentNo ratings yet
- Anaphylaxis: Copperbelt University School of Medicine MBCHB Pharmacology February 2015 (DR Sindwa Kanyimba)Document12 pagesAnaphylaxis: Copperbelt University School of Medicine MBCHB Pharmacology February 2015 (DR Sindwa Kanyimba)Homeground entertainmentNo ratings yet
- Anti-Tussives-Mucolytics-Expectorants-Feb 2015Document10 pagesAnti-Tussives-Mucolytics-Expectorants-Feb 2015Homeground entertainmentNo ratings yet
- Drug Treatment of Bronchial Asthma-Feb 2015Document39 pagesDrug Treatment of Bronchial Asthma-Feb 2015Homeground entertainmentNo ratings yet
- Principles of Diet Diversity and Dietary Modification - 2Document46 pagesPrinciples of Diet Diversity and Dietary Modification - 2Homeground entertainmentNo ratings yet
- Lecture 4 Pancreatic Hormones and DMDocument56 pagesLecture 4 Pancreatic Hormones and DMHomeground entertainmentNo ratings yet
- P9 Disorders of The CerebellumDocument29 pagesP9 Disorders of The CerebellumHomeground entertainmentNo ratings yet
- p5 Basal GangliaDocument18 pagesp5 Basal GangliaHomeground entertainmentNo ratings yet
- P6 Disorders of The Basal GangliaDocument12 pagesP6 Disorders of The Basal GangliaHomeground entertainmentNo ratings yet
- Lecture 2 Hearing and Equilibrium PhysiologyDocument52 pagesLecture 2 Hearing and Equilibrium PhysiologyHomeground entertainment100% (1)
- p8 The Physiology of The Flocculonodular LobeDocument16 pagesp8 The Physiology of The Flocculonodular LobeHomeground entertainmentNo ratings yet
- p4 Extra Pyramidal SystemDocument19 pagesp4 Extra Pyramidal SystemHomeground entertainmentNo ratings yet
- Copperbelt University School of Medicine Course: Physiology Course Code: Mbs 310Document5 pagesCopperbelt University School of Medicine Course: Physiology Course Code: Mbs 310Homeground entertainmentNo ratings yet
- The Copperbelt UniversityDocument5 pagesThe Copperbelt UniversityHomeground entertainmentNo ratings yet
- Lecture 3 - Thyroid Metabolic Hormones FMDocument57 pagesLecture 3 - Thyroid Metabolic Hormones FMHomeground entertainment100% (1)
- Charity Kapenda Muselema: Term 3 - NeurophysiologyDocument50 pagesCharity Kapenda Muselema: Term 3 - NeurophysiologyHomeground entertainmentNo ratings yet
- Aq27 Mechanism of OlfactionDocument5 pagesAq27 Mechanism of OlfactionHomeground entertainmentNo ratings yet
- Lecture 1 Principles of EndocrinologyDocument82 pagesLecture 1 Principles of EndocrinologyHomeground entertainmentNo ratings yet
- Lecture 2 - Pituitary Hormones and Their Control by The HypothalamusDocument39 pagesLecture 2 - Pituitary Hormones and Their Control by The HypothalamusHomeground entertainmentNo ratings yet
- Hypothalamus Regulation of Hormonal FunctionDocument47 pagesHypothalamus Regulation of Hormonal FunctionHomeground entertainmentNo ratings yet
- Underground Clinical Vignettes - BiochemistryDocument114 pagesUnderground Clinical Vignettes - Biochemistrydrksahil100% (2)
- Mousab Kibreet Notes of Internal Medicine 2ed EditionDocument253 pagesMousab Kibreet Notes of Internal Medicine 2ed EditionAbdulltif AliNo ratings yet
- EL 108 Midterm Module 1Document22 pagesEL 108 Midterm Module 1Ella Marie Gamayon PoloNo ratings yet
- Chapter 47 - Endocrine SystemDocument3 pagesChapter 47 - Endocrine SystemStacey100% (1)
- Np4 - Diagnostic ExamDocument26 pagesNp4 - Diagnostic ExamKhrisha Anne DavilloNo ratings yet
- Diagnostic Use of Facial Image Analysis Software in Endocrine and Genetic Disorders: Review, Current Results and Future PerspectivesDocument6 pagesDiagnostic Use of Facial Image Analysis Software in Endocrine and Genetic Disorders: Review, Current Results and Future PerspectivesRavi KiranNo ratings yet
- Sign & Symptoms: Disusun Oleh: Izri Miftahul AudryDocument12 pagesSign & Symptoms: Disusun Oleh: Izri Miftahul AudryIzri Miftahul AudryNo ratings yet
- Changing Faces: A Case-Based Review of Acromegaly: Special Care DentistryDocument5 pagesChanging Faces: A Case-Based Review of Acromegaly: Special Care DentistryZainab HajiNo ratings yet
- Usmle SuperDocument239 pagesUsmle SupermaksventileNo ratings yet
- Med Surg Exam 3 Comprehensive Review of The Material Covered For Professor Martinez Medical PDFDocument53 pagesMed Surg Exam 3 Comprehensive Review of The Material Covered For Professor Martinez Medical PDFBirhanu Ayenew100% (1)
- Hipofisis 3Document23 pagesHipofisis 3RafaelPetitNo ratings yet
- Nursing Practice Test - EndocrineDocument20 pagesNursing Practice Test - Endocrinemay17sanchez100% (7)
- Disorders of Pituitary GlandDocument34 pagesDisorders of Pituitary GlandninaaltheaNo ratings yet
- OctreotideDocument12 pagesOctreotiderpadmakrishnanNo ratings yet
- Ancient Maladies: An Exploration of Disease and Pathophysiology in Tanach and The TalmudDocument3 pagesAncient Maladies: An Exploration of Disease and Pathophysiology in Tanach and The Talmudoutdash2No ratings yet
- AcromegalyDocument3 pagesAcromegalylambomacNo ratings yet
- Anterior Pituitary HormonesDocument46 pagesAnterior Pituitary Hormonespramod bhaleraoNo ratings yet
- Hormone Regulation 1Document20 pagesHormone Regulation 1Manila MedNo ratings yet
- EndocrineDocument144 pagesEndocrineaartiNo ratings yet
- Diagnosis and Management of Pituitary TumorsDocument496 pagesDiagnosis and Management of Pituitary TumorsDavid EscamillaNo ratings yet
- Junnila 2015Document8 pagesJunnila 2015Andrei MarianNo ratings yet
- Endocrine System BulletsDocument28 pagesEndocrine System Bulletswinner gift flowersNo ratings yet
- Pituitary Tumours - An OverviewDocument81 pagesPituitary Tumours - An OverviewRAVIRAJ GHORPADE BELGAUM ADVANCED NEUROSURGERYNo ratings yet
- Content 3Document128 pagesContent 3Mohamed AbbasNo ratings yet
- EBM - 5. Adrenal DisordersDocument101 pagesEBM - 5. Adrenal DisordersBRI KUNo ratings yet
- Musculoskeletal Manifestations of Systemic DiseasesDocument16 pagesMusculoskeletal Manifestations of Systemic Diseasesreem100% (1)