Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
26 viewsAmino Acids: M. Zaharna Clin. Chem. 2009
Amino Acids: M. Zaharna Clin. Chem. 2009
Uploaded by
Ahmed GaberAmino acids are the building blocks of proteins. The 20 standard amino acids vary in their size, charge, and other properties, allowing proteins to take on diverse structures and functions. Humans can synthesize 11 of the 20 amino acids and must obtain the other 9, called "essential amino acids", through diet. Inherited disorders in amino acid metabolism, called aminoacidopathies, can cause health issues if left untreated. Phenylketonuria (PKU) is caused by a defect in the enzyme that breaks down phenylalanine, leading to its accumulation and risks of intellectual disability if not managed through diet. Newborns are screened for PKU and other aminoacidopathies through tests of blood or urine
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You might also like
- Preclinical Biochemistry and Medical Genetics Review 2023: For USMLE Step 1 and COMLEX-USA Level 1From EverandPreclinical Biochemistry and Medical Genetics Review 2023: For USMLE Step 1 and COMLEX-USA Level 1No ratings yet
- Astm E34-2011Document3 pagesAstm E34-2011Makarius YanuantoNo ratings yet
- AminoacidopathyDocument44 pagesAminoacidopathydrsoker2012No ratings yet
- Clinical FirstDocument342 pagesClinical FirstYasmeen AtiehNo ratings yet
- Amino Acidss & AminoacidopathiesDocument55 pagesAmino Acidss & AminoacidopathiesMustafa KhandgawiNo ratings yet
- Cch10 Protein2Document52 pagesCch10 Protein2Habtamu MollaNo ratings yet
- Proteins: M. Zaharna Ckin. Chem. 2009Document28 pagesProteins: M. Zaharna Ckin. Chem. 2009Ahmed GaberNo ratings yet
- Metabolism of Individual Amino Acids and Biosynthesis ofDocument33 pagesMetabolism of Individual Amino Acids and Biosynthesis ofAboubakar Moalim Mahad moh'dNo ratings yet
- 4 Protein ReviewDocument87 pages4 Protein Reviewmika de guzmanNo ratings yet
- Lesson 8 - Amino AcidDocument10 pagesLesson 8 - Amino Acidchristian Jay HorseradaNo ratings yet
- Chemical Pathology BS-MLT 5Th SemesterDocument36 pagesChemical Pathology BS-MLT 5Th SemesterMuhammad AbdullahNo ratings yet
- Chapter X - Mechanism of Protein MetabolismDocument30 pagesChapter X - Mechanism of Protein MetabolismAngelo AngelesNo ratings yet
- Amino Acids Metabolism Final For Pharm 2014Document57 pagesAmino Acids Metabolism Final For Pharm 2014Getu LuchesaNo ratings yet
- Proteins and Liver Function TestsDocument56 pagesProteins and Liver Function TestsjoanNo ratings yet
- A3.Proteins 1Document46 pagesA3.Proteins 1ÇağlaNo ratings yet
- Antimalarial DrugsDocument36 pagesAntimalarial DrugsKasim UmarNo ratings yet
- TryptophanDocument41 pagesTryptophanmahalakshmiNo ratings yet
- ProteinDocument67 pagesProteinSri DeviNo ratings yet
- Digestion Absorpton of ProteinsDocument37 pagesDigestion Absorpton of Proteinsmohammed aliNo ratings yet
- Week 4 Roles of ProteinDocument40 pagesWeek 4 Roles of ProteinPrincess Joy YabutNo ratings yet
- 1 Overview & DigestionAbsorption Protein MetabolismDocument37 pages1 Overview & DigestionAbsorption Protein MetabolismAshish K JoyNo ratings yet
- Lecture 5 - Synthesis of Non-Essential AADocument17 pagesLecture 5 - Synthesis of Non-Essential AAciyace7849No ratings yet
- METABOLISMDocument18 pagesMETABOLISMLT DRAGONXNo ratings yet
- Non - Protein Nitrogen Compounds-1Document71 pagesNon - Protein Nitrogen Compounds-1reuben kwotaNo ratings yet
- Protein and Amino Acids: Metabolism and AnalysisDocument35 pagesProtein and Amino Acids: Metabolism and AnalysisWindi MoseNo ratings yet
- Amino AcidsDocument57 pagesAmino AcidsRAMA ABO SAMRANo ratings yet
- 3 CompoundsDocument27 pages3 CompoundsfidhavfathimaNo ratings yet
- Presentasi AmphibiaDocument30 pagesPresentasi Amphibiabima satria moektiNo ratings yet
- Pancreas Function MazenDocument15 pagesPancreas Function MazenAhmed GaberNo ratings yet
- Xenobiotics 2022Document22 pagesXenobiotics 2022Leul DawitNo ratings yet
- Aminoacidopathies OCT2008 - Clinical Chem Lect 3rd Yr MT - 1st SemesterDocument19 pagesAminoacidopathies OCT2008 - Clinical Chem Lect 3rd Yr MT - 1st SemesterburdihanNo ratings yet
- Wa0028.Document46 pagesWa0028.Ziyadan AtiqueNo ratings yet
- 6 Proteins Their Digestion & AbsorptionsDocument18 pages6 Proteins Their Digestion & AbsorptionshodaNo ratings yet
- ProteinDocument49 pagesProteinsamuel mergaNo ratings yet
- 1 - AAs - Disposal of Nitrogen-2022Document87 pages1 - AAs - Disposal of Nitrogen-2022Ayman RamadanNo ratings yet
- 7-8. Metabolism of Amino Acids. Catabolism of Individual Amino Acids. Amino Acid Derivatives, Special ProductsDocument87 pages7-8. Metabolism of Amino Acids. Catabolism of Individual Amino Acids. Amino Acid Derivatives, Special ProductsErin HillNo ratings yet
- Protein FoldingDocument21 pagesProtein FoldingMayank SNo ratings yet
- Clinchem 1565Document4 pagesClinchem 1565ghfkhgfjhfgNo ratings yet
- Enzymes Ppt-NandanaDocument26 pagesEnzymes Ppt-NandanaLingaraj Kumar100% (1)
- Classification and The Chemistry of Pharmaceutical Products The Top Ten Drugs 1. Lipitor® (Atorvastatin)Document15 pagesClassification and The Chemistry of Pharmaceutical Products The Top Ten Drugs 1. Lipitor® (Atorvastatin)Yong LiNo ratings yet
- Hyper AmmoniaDocument10 pagesHyper AmmoniaThamizh Arasi Vinayagam100% (1)
- Ni Nyoman Ayu Dewi Dept. of Biochemistry, Faculty of Medicine Udayana University Ayu - Dewi@unud - Ac.idDocument37 pagesNi Nyoman Ayu Dewi Dept. of Biochemistry, Faculty of Medicine Udayana University Ayu - Dewi@unud - Ac.idWida Utami100% (1)
- Quazi VitaminsDocument128 pagesQuazi VitaminsNaji Mohamed AlfatihNo ratings yet
- Theory766278 1Document23 pagesTheory766278 1Shirin GulNo ratings yet
- Enzymes: M. Zaharna Clin. Chem. 2009Document32 pagesEnzymes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Urea CycleDocument42 pagesUrea CycleDeea LobonțiuNo ratings yet
- كيمياء سريرية 16Document15 pagesكيمياء سريرية 16Aya AshrafNo ratings yet
- Cytochrome p450 InteractionDocument9 pagesCytochrome p450 Interactionkondaveeti sreenivasulu NaiduNo ratings yet
- Metabolism of ProteinsDocument50 pagesMetabolism of ProteinsAbdur RehmanNo ratings yet
- Lesson 6 Chemical Examination of UrineDocument66 pagesLesson 6 Chemical Examination of UrineFaith TambongNo ratings yet
- Lecture 5Document23 pagesLecture 5bkrmnbxbjtNo ratings yet
- Lecture 3 Urea Cycle DisordersDocument27 pagesLecture 3 Urea Cycle Disordersamjadm2002No ratings yet
- Amino Acid Oxidation andDocument23 pagesAmino Acid Oxidation andRahma FauziahNo ratings yet
- Proteins MetabolismDocument27 pagesProteins MetabolismFouzia GillNo ratings yet
- Amino Acid MetabolismDocument29 pagesAmino Acid MetabolismERIAS TENYWANo ratings yet
- Investigation of Plasma Protein DisordersDocument10 pagesInvestigation of Plasma Protein DisordersJosiah BimabamNo ratings yet
- Protein Metabolism: by Dr. Mustafa Kahtan Al-BayatyDocument37 pagesProtein Metabolism: by Dr. Mustafa Kahtan Al-BayatyIraqiNo ratings yet
- Cyp 450Document32 pagesCyp 450kondaveeti sreenivasulu NaiduNo ratings yet
- HyperammonemiaDocument10 pagesHyperammonemiaAsfoor gake1No ratings yet
- Lect1 - 2017Document28 pagesLect1 - 2017George MakoriNo ratings yet
- امتحان برومتريك ميكروبيولوجي 19-11-2011Document7 pagesامتحان برومتريك ميكروبيولوجي 19-11-2011Ahmed GaberNo ratings yet
- هام جداDocument93 pagesهام جداAhmed GaberNo ratings yet
- نماذج اسئلة الهيئة السعودية للتخصصات الصحية للاخصائيين والاطباء بالمختبراتDocument68 pagesنماذج اسئلة الهيئة السعودية للتخصصات الصحية للاخصائيين والاطباء بالمختبراتAhmed GaberNo ratings yet
- Non-Protein Nitrogen (NPN) CompoundsDocument34 pagesNon-Protein Nitrogen (NPN) CompoundsAhmed GaberNo ratings yet
- Liver Function Mazen 1Document24 pagesLiver Function Mazen 1Ahmed GaberNo ratings yet
- Lipids - & - Lipoproteins - Mazen 2Document31 pagesLipids - & - Lipoproteins - Mazen 2Ahmed GaberNo ratings yet
- Pancreas Function MazenDocument15 pagesPancreas Function MazenAhmed GaberNo ratings yet
- Overview of Clinical ChemistryDocument30 pagesOverview of Clinical ChemistryAhmed Gaber0% (1)
- Enzymes: M. Zaharna Clin. Chem. 2009Document32 pagesEnzymes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Enzymes: M. Zaharna Clin. Chem. 2009Document33 pagesEnzymes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Electrolytes: M. Zaharna Clin. Chem. 2009Document20 pagesElectrolytes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Electrolytes: M. Zaharna Clin. Chem. 2009Document35 pagesElectrolytes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Carbohydrates - Part - 1 - MazenDocument35 pagesCarbohydrates - Part - 1 - MazenAhmed GaberNo ratings yet
- Proteins 1: M. Zaharna Clini. Chem. 2009Document50 pagesProteins 1: M. Zaharna Clini. Chem. 2009Ahmed GaberNo ratings yet
- Carbohydrates - Part - 2 - MazenDocument28 pagesCarbohydrates - Part - 2 - MazenAhmed GaberNo ratings yet
- Blood Gases Mazen 2Document16 pagesBlood Gases Mazen 2Ahmed GaberNo ratings yet
- " Chest:: by Definition Malignant Tumor of The Lung Primary Its EtiologyDocument10 pages" Chest:: by Definition Malignant Tumor of The Lung Primary Its EtiologyAhmed GaberNo ratings yet
- 1 - MCQs Classified اسئله د محمد امامDocument68 pages1 - MCQs Classified اسئله د محمد امامAhmed GaberNo ratings yet
- Proteins: M. Zaharna Ckin. Chem. 2009Document28 pagesProteins: M. Zaharna Ckin. Chem. 2009Ahmed GaberNo ratings yet
- Blood Gases, PH, and Buffer SystemDocument22 pagesBlood Gases, PH, and Buffer SystemAhmed GaberNo ratings yet
- Synthetic Approaches Towards Tubulysins and Derivatives ThereofDocument19 pagesSynthetic Approaches Towards Tubulysins and Derivatives ThereofNgô Tuấn KiệtNo ratings yet
- Worksheet 11 KeyDocument8 pagesWorksheet 11 KeyNguyễn Minh AnhNo ratings yet
- Pah - DB Eupah - 5990-4883enDocument6 pagesPah - DB Eupah - 5990-4883enridermateNo ratings yet
- Uponor Push 23b Shunt Med Wilo Pumpe MANDocument20 pagesUponor Push 23b Shunt Med Wilo Pumpe MANschritte1No ratings yet
- MSDS ChevronnDocument9 pagesMSDS ChevronnHasan UğurluNo ratings yet
- Industrial PharmacyDocument9 pagesIndustrial PharmacyMr nobodyNo ratings yet
- Oxygen Dissolved 4500Document3 pagesOxygen Dissolved 4500Sandra Vanessa Mejia SantillanNo ratings yet
- Secrets of IITDocument5 pagesSecrets of IITAnil Kumar VermaNo ratings yet
- Science q1m2Document30 pagesScience q1m2Juana Isabel B. LunaNo ratings yet
- D and F Block ElementsDocument8 pagesD and F Block ElementsPrashanth SNo ratings yet
- Bellano Digital LaminatesDocument56 pagesBellano Digital LaminatesMital DamaniNo ratings yet
- COQ Exxon-Intertek Maret 19Document1 pageCOQ Exxon-Intertek Maret 19satriasinagaNo ratings yet
- Chemical Moles & Formulae Review 2 (08.07.21)Document4 pagesChemical Moles & Formulae Review 2 (08.07.21)Micheelle JeannethNo ratings yet
- Brochure - Producedwater - Sorbwater - FOR WEBDocument12 pagesBrochure - Producedwater - Sorbwater - FOR WEBjuan vazquezNo ratings yet
- The Mole Concept: Learning CompetencyDocument14 pagesThe Mole Concept: Learning Competencylevi0417No ratings yet
- Bonding (Diamond, Graphite, Fullerene and Silicon-Dioxide)Document1 pageBonding (Diamond, Graphite, Fullerene and Silicon-Dioxide)Safe GuardNo ratings yet
- 15-TMSS-06 R.0Document13 pages15-TMSS-06 R.0wastazoheb_700349353No ratings yet
- Chemsheets As 1005 Ionisation EnergiesDocument2 pagesChemsheets As 1005 Ionisation Energiesangel ranaNo ratings yet
- Lowmain Tech ManualDocument30 pagesLowmain Tech ManualArunava BasakNo ratings yet
- Module 1 Long QuizDocument3 pagesModule 1 Long QuizJOHANNA RACHEL VILLASISNo ratings yet
- Cell Biology: Course Code: LSE-01 Assignment Code: LSE-01/TMA/2020 Maximum Marks: 100Document26 pagesCell Biology: Course Code: LSE-01 Assignment Code: LSE-01/TMA/2020 Maximum Marks: 100Rajni KumariNo ratings yet
- Repair: Re Pair in Single-Skin ConstructionDocument15 pagesRepair: Re Pair in Single-Skin ConstructionMirceaNo ratings yet
- Boiler Feed Water and Steam ChemistryDocument4 pagesBoiler Feed Water and Steam ChemistryVajid MadathilNo ratings yet
- 12th Physics Structure of Atoms & Nuclei Notes in EnglishDocument82 pages12th Physics Structure of Atoms & Nuclei Notes in EnglishAman Singh RaoNo ratings yet
- US8961680Document12 pagesUS8961680subramanian.sNo ratings yet
- Karnataka - Notified Protection Officers: (District Level)Document35 pagesKarnataka - Notified Protection Officers: (District Level)astuteNo ratings yet
- Charaterization of Liquid Solid ReactionDocument8 pagesCharaterization of Liquid Solid ReactionYuni_Arifwati_5495No ratings yet
- Pharma Manual PDFDocument24 pagesPharma Manual PDFLawrence Agada88% (8)
- Ion Exchange With Natural Zeolites: An Alternative For Water Softening?Document7 pagesIon Exchange With Natural Zeolites: An Alternative For Water Softening?Yana ElzyNo ratings yet
Amino Acids: M. Zaharna Clin. Chem. 2009
Amino Acids: M. Zaharna Clin. Chem. 2009
Uploaded by
Ahmed Gaber0 ratings0% found this document useful (0 votes)
26 views39 pagesAmino acids are the building blocks of proteins. The 20 standard amino acids vary in their size, charge, and other properties, allowing proteins to take on diverse structures and functions. Humans can synthesize 11 of the 20 amino acids and must obtain the other 9, called "essential amino acids", through diet. Inherited disorders in amino acid metabolism, called aminoacidopathies, can cause health issues if left untreated. Phenylketonuria (PKU) is caused by a defect in the enzyme that breaks down phenylalanine, leading to its accumulation and risks of intellectual disability if not managed through diet. Newborns are screened for PKU and other aminoacidopathies through tests of blood or urine
Original Description:
Original Title
Amino_Acids_----Mazen
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentAmino acids are the building blocks of proteins. The 20 standard amino acids vary in their size, charge, and other properties, allowing proteins to take on diverse structures and functions. Humans can synthesize 11 of the 20 amino acids and must obtain the other 9, called "essential amino acids", through diet. Inherited disorders in amino acid metabolism, called aminoacidopathies, can cause health issues if left untreated. Phenylketonuria (PKU) is caused by a defect in the enzyme that breaks down phenylalanine, leading to its accumulation and risks of intellectual disability if not managed through diet. Newborns are screened for PKU and other aminoacidopathies through tests of blood or urine
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
Download as ppt, pdf, or txt
0 ratings0% found this document useful (0 votes)
26 views39 pagesAmino Acids: M. Zaharna Clin. Chem. 2009
Amino Acids: M. Zaharna Clin. Chem. 2009
Uploaded by
Ahmed GaberAmino acids are the building blocks of proteins. The 20 standard amino acids vary in their size, charge, and other properties, allowing proteins to take on diverse structures and functions. Humans can synthesize 11 of the 20 amino acids and must obtain the other 9, called "essential amino acids", through diet. Inherited disorders in amino acid metabolism, called aminoacidopathies, can cause health issues if left untreated. Phenylketonuria (PKU) is caused by a defect in the enzyme that breaks down phenylalanine, leading to its accumulation and risks of intellectual disability if not managed through diet. Newborns are screened for PKU and other aminoacidopathies through tests of blood or urine
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
Download as ppt, pdf, or txt
You are on page 1of 39
Amino Acids
M. Zaharna Clin. Chem. 2009
Introduction
• Amino acids play central roles both as building
blocks of proteins and as intermediates in
metabolism.
• The 20 amino acids that are found within
proteins convey a vast array of chemical
versatility.
• The precise amino acid content, and the
sequence of those amino acids, of a specific
protein, is determined by the sequence of the
bases in the gene that encodes that protein.
• The chemical properties of the amino acids of
proteins determine the biological activity of the
protein.
M. Zaharna Clin. Chem. 2009
Introduction
• In addition, proteins contain within their
amino acid sequences the necessary
information to
• determine how that protein will fold into a three
dimensional structure,
• and the stability of the resulting structure.
• It is important to keep in mind that one of the
more important reasons to understand amino
acid structure and properties is to be able to
understand protein structure and properties.
• The vastly complex characteristics of even a
small, relatively simple, protein are a
composite of the properties of the amino
acids which comprise the protein.
M. Zaharna Clin. Chem. 2009
Amino Acid Structure
• An α-amino acid consists of:
– a central carbon atom, called the α carbon,
– linked to an amino group,
– a carboxylic acid group,
– a hydrogen atom,
– and a distinctive R group.
• The R group is often referred to as the side
chain.
• The two mirror-image forms are called the L
isomer and the d isomer.
M. Zaharna Clin. Chem. 2009
L & D isomers
M. Zaharna Clin. Chem. 2009
• Twenty kinds of side chains varying in
size, shape, charge, hydrogen-bonding
capacity, hydrophobic character, and
chemical reactivity are commonly found.
• The remarkable range of functions
mediated by proteins results from the
diversity and versatility of these 20
building blocks.
M. Zaharna Clin. Chem. 2009
M. Zaharna Clin. Chem. 2009
Essential Amino Acids
• Humans can produce 11 of the 20 amino acids.
• The others must be supplied in the food.
• Failure to obtain enough of even 1 of the 9
essential amino acids, those that we cannot
make, results in degradation of the body's
proteins—muscle and so forth—to obtain the
one amino acid that is needed.
• Unlike fat and starch, the human body does not
store excess amino acids for later use—the
amino acids must be in the food every day.
M. Zaharna Clin. Chem. 2009
Metabolism
• Proteolytic enzymes such as pepsin and trypsin
digest dietary proteins into their constituent
amino acids, the breakdown of body proteins is
a source of amino acids.
• The amino acid pool is used for the synthesis of
body proteins e.g. plasma, intracellular and
structural proteins.
• Amino acids are also used for the synthesis of
nonprotein nitrogen "NPN" – containing
compounds such as purines, pyrimidines,
creatine, histamine, thyroxine and others
M. Zaharna Clin. Chem. 2009
• All tissues have some capability for synthesis of
the non-essential amino acids, and conversion
of non-amino acid carbon skeletons into amino
acids.
• However, the liver is the major site of nitrogen
metabolism in the body.
• In times of dietary surplus, the potentially toxic
nitrogen of amino acids is eliminated via
transaminations, deamination, and urea
formation;
• The carbon skeletons are generally conserved
as carbohydrate, via gluconeogenesis, or as
fatty acid via fatty acid synthesis pathways.
M. Zaharna Clin. Chem. 2009
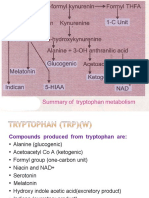
• In this respect amino acids fall into three
categories: glucogenic, ketogenic, or
glucogenic and ketogenic.
• Glucogenic amino acids are those that give
rise to a net production of pyruvate or TCA
cycle intermediates, such as α-ketoglutarate
or oxaloacetate, (Alanine can be deaminated
to pyruvate, arginine to – ketoglutarate )
• Lysine and leucine are the only amino acids
that are solely ketogenic, giving rise only to
acetylCoA or acetoacetylCoA.
M. Zaharna Clin. Chem. 2009
• A small group of amino acids such as
phenylalanine, and tyrosine give rise to
both glucose and fatty acid precursors and
are thus characterized as being
glucogenic and ketogenic.
M. Zaharna Clin. Chem. 2009
M. Zaharna Clin. Chem. 2009
Aminoacidopathies
• They are rare inherited disorders of amino
acid metabolism.
• Hereditary disorders of amino acid processing
can be the result of :
– defects either in the breakdown of amino acids
(activity of a specific enzyme )
– or in the body's ability to get the amino acids into
cells (membrane transport system ).
• More than 100 diseases have been identified
that result from inborn errors of amino acid
metabolism.
• Because these disorders produce symptoms
early in life, newborns are routinely screened
for several common ones.
M. Zaharna Clin. Chem. 2009
• Phenylketonuria (PKU)
• Tyrosinemia
• Alkaptonuria
• Maple syrup urine disease
• Isovaleric Acidemia
• Homocystinuria
• Cystinuria
M. Zaharna Clin. Chem. 2009
Phenylketonuria (PKU)
• Phenylalanine hydroxylase is
an enzyme which converts
phenylalanine to tyrosine.
• A deficiency of this enzyme
leads to a buildup of
phenylalanine which results in
severe mental retardation.
• Phenylalanine accumulates and is metabolized
by alternate degradative pathway into
phenylpyruvic acid and others leading to mental
retardation.
M. Zaharna Clin. Chem. 2009
• This condition, known
as phenylketonuria
(PKU), is an
autosomal recessive
inborn error of
metabolism.
M. Zaharna Clin. Chem. 2009
Testing for PKU
• Testing is done either on the serum (Guthrie test) or on
the urine.
• Testing is not valid until the newborn has ingested an
ample amount of the amino acid phenylalanine, which is
found in human and cow’s milk.
• Two or three days of intake are usually sufficient for the
Guthrie test.
• Urine PKU testing is usually done after the infant is 4 to 6
weeks old.
• Normal blood phenylalanine level is about 1.2 - 3.4 mg/dl.
In PKU, levels may range from 6 to 80mg/dl, usually
greater than 30mg/dl
M. Zaharna Clin. Chem. 2009
Guthrie bacterial inhibition assay
• Spores of B. Subtilis are incorporated into
an agar plate that contains 2-
thienylalanine, a metabolic antagonist to
B. Subtilis growth.
• A filter paper disk impregnated with blood
from the infant is placed on the agar.
• If the blood level of phenylalanine
exceeds a range of 2 – 4 mg/dl, the
phenylalanine counteracts the antagonist,
and the bacterial growth occurs.
M. Zaharna Clin. Chem. 2009
Guthrie bacterial inhibition assay
• The infant must be at least 24h of
age to ensure adequate time for
enzyme and amino acid levels to
develop.
• The sample should be taken before
the administration of antibiotics or
transfusion of blood.
• Premature infants can show false-
positive results due to the
immaturity of the liver's enzyme
systems.
M. Zaharna Clin. Chem. 2009
Microfluorometric assay
• For the direct measurement of phenylalanine in
dried blood filter discs. It yields quantitative
results, not affected by the presence of antibiotics.
• The procedure is based on the fluorescence of a
complex formed of phenylalanine-ninhydrin-
copper in the presence of a dipeptide "l-leucyl-l-
alanine".
• The procedure starts with extraction using
trichloroacetic acid, then a mixture of ninhydrin,
succinate, leucylalanine and copper tartarate is
added, the fluorescence of the complex is
measured using excitation/emission wavelengths
of 360nm and 530nm respectively.
M. Zaharna Clin. Chem. 2009
Reference method
• The reference method for quantitative
serum phenylalanine is HPLC.
• The normal limits of serum
phenylalanine levels of full term normal
weight newborns range from 1.2 mg/dl
– 3.4 mg/dl.
M. Zaharna Clin. Chem. 2009
Urine testing for phenylpyruvic
acid
• Used for diagnosis in questionable
cases and for monitoring of dietary
therapy.
• It involves the reaction of ferric chloride
with phenylpyruvic acid in urine to
produce a green color.
M. Zaharna Clin. Chem. 2009
Prenatal diagnosis and detection
of carrier status
• In families with PKU, testing is now
available using DNA analysis.
• The test is based on revealing multiple
independent mutations at the
phenylalanine hydroxylase locus.
M. Zaharna Clin. Chem. 2009
Tyrosinemia
• Tyrosinemia is a genetic disorder
characterized by elevated blood levels of the
amino acid tyrosine,
• Tyrosine is a building block of most proteins.
• Tyrosinemia is caused by the shortage
(deficiency) of one of the enzymes required
for the multistep process that breaks down
tyrosine.
• If untreated, tyrosine and its byproducts build
up in tissues and organs, which leads to
serious medical problems.
M. Zaharna Clin. Chem. 2009
Tyrosinemia
• There are three types of tyrosinemia. Each
has distinctive symptoms and is caused by
the deficiency of a different enzyme.
• Type I tyrosinemia, the most severe form of
this disorder, is caused by a shortage of the
enzyme fumarylacetoacetate hydrolase.
• Type II tyrosinemia is caused by a deficiency
of the enzyme tyrosine aminotransferase.
M. Zaharna Clin. Chem. 2009
Alkaptonuria
• It is due the deficiency of homogentistate
oxidase in the tyrosine catabolic pathway.
• Accumulation of homogentisic acid in urine
causes its darkening upon exposure to a
atmosphere.
• Alkaptonuric patients have no immediate
problems, but later high levels of
homogentisic acid gradually accumulate in
connective tissue, causing generalized
pigmentation of these tissues and an arthritis
like degeneration.
M. Zaharna Clin. Chem. 2009
M. Zaharna Clin. Chem. 2009
Maple Syrup Urine Disease
"MSUD"
• Maple syrup urine disease is an inherited disorder
in which the body is unable to process certain
amino acids properly. The condition gets its name
from the distinctive sweet odor of affected infants'
urine.
• Mutations in 4 genes cause maple syrup urine
disease.
• These four genes provide instructions for making
proteins that work together as a complex.
• The protein complex is essential for breaking
down the amino acids leucine, isoleucine, and
valine, which are present in many kinds of food
M. Zaharna Clin. Chem. 2009
• As a result, these amino acids and their
byproducts build up in the body.
• Because high levels of these substances are
toxic to the brain and other organs, their
accumulation leads to the serious medical
problems.
M. Zaharna Clin. Chem. 2009
Tests
• A modified Guthrie test is used for neonatal
screening. The metabolic inhibitor of B. Subtilis is 4-
azaleucine.
• Positive test for MUSD, elevated level of leucine
from a filter paper disc impregnated with infants
blood will overcome the inhibitor and bacterial
growth occurs.
• Confirmed diagnosis is based on finding increased
levels of the three amino acids in plasma and urine
with leucine being in highest concentration.
• A leucine level above 4 mg/dl is indicative of MUSD.
• MUSD can be diagnosed prenatally by measuring
the decarboxylase enzyme concentration in cells
cultured from amniotic fluid.
M. Zaharna Clin. Chem. 2009
Isovaleric Acidemia
• It results from a deficiency of the
enzyme isovaleryl-COA
dehydrogenase in the degradative
pathway of leucine.
• The elevated isovalerylglycine levels
can be identified by chromatography.
M. Zaharna Clin. Chem. 2009
Homocystinuria
• Homocysteine is an intermediate amino acid
in the synthesis of cysteine from
menthionine.
• Homocystinuria is caused by the impaired
activity of the enzyme cystathionine -
synthase which results in elevated plasma
and urine levels of homocysteine and
methionine.
• Newborns show no abnormalities, physical
defects develop gradually with age.
• Clinical findings in late childhood include
thrombosis, osteoporosis, dislocated eye
lenses due to the lack of cysteine synthesis
which is essential for collagen formation.
M. Zaharna Clin. Chem. 2009
M. Zaharna Clin. Chem. 2009
• Neonatal screening with a Guthrie test
using L-methionine sulfoximine as the
metabolic inhibitor. Increased plasma
methionine levels from affected infants
will result in bacterial growth.
• Elevations in urinary homocystine can
be detected by the cyanide-
nitroprusside spot test.
M. Zaharna Clin. Chem. 2009
Cystinuria
• It is caused by a defect in the amino acid transport
system rather than a metabolic enzyme deficiency.
• Normally, amino acids are free filtered by the glomerulus
and then actively reabsorbed in the proximal renal
tubules.
• In cystinuria, there is a 20-30 fold increase in the urinary
excretion of cystine due to a genetic defect in the renal
resorptive mechanism.
• Because cystine is relatively insoluble, it tends to
precipitate in the kidney tubules and form urinary calculi.
• Cystinuria can be tested by cyanide-nitroprusside test.
M. Zaharna Clin. Chem. 2009
Amino Acid Analysis
• Blood samples drawn after 6-8 h fasting to
avoid the effect of absorbed amino acids
originating from dietary proteins.
• The sample is collected in heparin and the
plasma is promptly removed from the cells
"as cells contain higher concentration of
amino acids", hemolysis should be avoided
for the same reason.
• Deproteinization within 30 min of collection,
analysis should be performed immediately or
the sample should be stored at -20OC to
-40OC.
M. Zaharna Clin. Chem. 2009
• Urinary amino acid analysis can be
performed on a random specimen for
screening purposes, but for quantitation, a
24 h urine preserved with thymol or organic
solvent is required. Amniotic fluid also may
be analyzed.
• The method of choice is the two-dimensional
chromatography, the amino acids are
allowed to migrate along one solvent front,
and then the chromatogram is rotated 90O
and a second solvent migration occurs.
M. Zaharna Clin. Chem. 2009
• The chromatogram is visualized by
staining with ninhydrin, which gives a
blue color with most amino acids.
• Confirmatory test for an amino acid
disorder include separation and
quantitation by cation-exchange
chromatography using a gradient buffer
elution.
• HPLC reversed-phase system
equipped with fluorescence detection is
another choice.
M. Zaharna Clin. Chem. 2009
You might also like
- Preclinical Biochemistry and Medical Genetics Review 2023: For USMLE Step 1 and COMLEX-USA Level 1From EverandPreclinical Biochemistry and Medical Genetics Review 2023: For USMLE Step 1 and COMLEX-USA Level 1No ratings yet
- Astm E34-2011Document3 pagesAstm E34-2011Makarius YanuantoNo ratings yet
- AminoacidopathyDocument44 pagesAminoacidopathydrsoker2012No ratings yet
- Clinical FirstDocument342 pagesClinical FirstYasmeen AtiehNo ratings yet
- Amino Acidss & AminoacidopathiesDocument55 pagesAmino Acidss & AminoacidopathiesMustafa KhandgawiNo ratings yet
- Cch10 Protein2Document52 pagesCch10 Protein2Habtamu MollaNo ratings yet
- Proteins: M. Zaharna Ckin. Chem. 2009Document28 pagesProteins: M. Zaharna Ckin. Chem. 2009Ahmed GaberNo ratings yet
- Metabolism of Individual Amino Acids and Biosynthesis ofDocument33 pagesMetabolism of Individual Amino Acids and Biosynthesis ofAboubakar Moalim Mahad moh'dNo ratings yet
- 4 Protein ReviewDocument87 pages4 Protein Reviewmika de guzmanNo ratings yet
- Lesson 8 - Amino AcidDocument10 pagesLesson 8 - Amino Acidchristian Jay HorseradaNo ratings yet
- Chemical Pathology BS-MLT 5Th SemesterDocument36 pagesChemical Pathology BS-MLT 5Th SemesterMuhammad AbdullahNo ratings yet
- Chapter X - Mechanism of Protein MetabolismDocument30 pagesChapter X - Mechanism of Protein MetabolismAngelo AngelesNo ratings yet
- Amino Acids Metabolism Final For Pharm 2014Document57 pagesAmino Acids Metabolism Final For Pharm 2014Getu LuchesaNo ratings yet
- Proteins and Liver Function TestsDocument56 pagesProteins and Liver Function TestsjoanNo ratings yet
- A3.Proteins 1Document46 pagesA3.Proteins 1ÇağlaNo ratings yet
- Antimalarial DrugsDocument36 pagesAntimalarial DrugsKasim UmarNo ratings yet
- TryptophanDocument41 pagesTryptophanmahalakshmiNo ratings yet
- ProteinDocument67 pagesProteinSri DeviNo ratings yet
- Digestion Absorpton of ProteinsDocument37 pagesDigestion Absorpton of Proteinsmohammed aliNo ratings yet
- Week 4 Roles of ProteinDocument40 pagesWeek 4 Roles of ProteinPrincess Joy YabutNo ratings yet
- 1 Overview & DigestionAbsorption Protein MetabolismDocument37 pages1 Overview & DigestionAbsorption Protein MetabolismAshish K JoyNo ratings yet
- Lecture 5 - Synthesis of Non-Essential AADocument17 pagesLecture 5 - Synthesis of Non-Essential AAciyace7849No ratings yet
- METABOLISMDocument18 pagesMETABOLISMLT DRAGONXNo ratings yet
- Non - Protein Nitrogen Compounds-1Document71 pagesNon - Protein Nitrogen Compounds-1reuben kwotaNo ratings yet
- Protein and Amino Acids: Metabolism and AnalysisDocument35 pagesProtein and Amino Acids: Metabolism and AnalysisWindi MoseNo ratings yet
- Amino AcidsDocument57 pagesAmino AcidsRAMA ABO SAMRANo ratings yet
- 3 CompoundsDocument27 pages3 CompoundsfidhavfathimaNo ratings yet
- Presentasi AmphibiaDocument30 pagesPresentasi Amphibiabima satria moektiNo ratings yet
- Pancreas Function MazenDocument15 pagesPancreas Function MazenAhmed GaberNo ratings yet
- Xenobiotics 2022Document22 pagesXenobiotics 2022Leul DawitNo ratings yet
- Aminoacidopathies OCT2008 - Clinical Chem Lect 3rd Yr MT - 1st SemesterDocument19 pagesAminoacidopathies OCT2008 - Clinical Chem Lect 3rd Yr MT - 1st SemesterburdihanNo ratings yet
- Wa0028.Document46 pagesWa0028.Ziyadan AtiqueNo ratings yet
- 6 Proteins Their Digestion & AbsorptionsDocument18 pages6 Proteins Their Digestion & AbsorptionshodaNo ratings yet
- ProteinDocument49 pagesProteinsamuel mergaNo ratings yet
- 1 - AAs - Disposal of Nitrogen-2022Document87 pages1 - AAs - Disposal of Nitrogen-2022Ayman RamadanNo ratings yet
- 7-8. Metabolism of Amino Acids. Catabolism of Individual Amino Acids. Amino Acid Derivatives, Special ProductsDocument87 pages7-8. Metabolism of Amino Acids. Catabolism of Individual Amino Acids. Amino Acid Derivatives, Special ProductsErin HillNo ratings yet
- Protein FoldingDocument21 pagesProtein FoldingMayank SNo ratings yet
- Clinchem 1565Document4 pagesClinchem 1565ghfkhgfjhfgNo ratings yet
- Enzymes Ppt-NandanaDocument26 pagesEnzymes Ppt-NandanaLingaraj Kumar100% (1)
- Classification and The Chemistry of Pharmaceutical Products The Top Ten Drugs 1. Lipitor® (Atorvastatin)Document15 pagesClassification and The Chemistry of Pharmaceutical Products The Top Ten Drugs 1. Lipitor® (Atorvastatin)Yong LiNo ratings yet
- Hyper AmmoniaDocument10 pagesHyper AmmoniaThamizh Arasi Vinayagam100% (1)
- Ni Nyoman Ayu Dewi Dept. of Biochemistry, Faculty of Medicine Udayana University Ayu - Dewi@unud - Ac.idDocument37 pagesNi Nyoman Ayu Dewi Dept. of Biochemistry, Faculty of Medicine Udayana University Ayu - Dewi@unud - Ac.idWida Utami100% (1)
- Quazi VitaminsDocument128 pagesQuazi VitaminsNaji Mohamed AlfatihNo ratings yet
- Theory766278 1Document23 pagesTheory766278 1Shirin GulNo ratings yet
- Enzymes: M. Zaharna Clin. Chem. 2009Document32 pagesEnzymes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Urea CycleDocument42 pagesUrea CycleDeea LobonțiuNo ratings yet
- كيمياء سريرية 16Document15 pagesكيمياء سريرية 16Aya AshrafNo ratings yet
- Cytochrome p450 InteractionDocument9 pagesCytochrome p450 Interactionkondaveeti sreenivasulu NaiduNo ratings yet
- Metabolism of ProteinsDocument50 pagesMetabolism of ProteinsAbdur RehmanNo ratings yet
- Lesson 6 Chemical Examination of UrineDocument66 pagesLesson 6 Chemical Examination of UrineFaith TambongNo ratings yet
- Lecture 5Document23 pagesLecture 5bkrmnbxbjtNo ratings yet
- Lecture 3 Urea Cycle DisordersDocument27 pagesLecture 3 Urea Cycle Disordersamjadm2002No ratings yet
- Amino Acid Oxidation andDocument23 pagesAmino Acid Oxidation andRahma FauziahNo ratings yet
- Proteins MetabolismDocument27 pagesProteins MetabolismFouzia GillNo ratings yet
- Amino Acid MetabolismDocument29 pagesAmino Acid MetabolismERIAS TENYWANo ratings yet
- Investigation of Plasma Protein DisordersDocument10 pagesInvestigation of Plasma Protein DisordersJosiah BimabamNo ratings yet
- Protein Metabolism: by Dr. Mustafa Kahtan Al-BayatyDocument37 pagesProtein Metabolism: by Dr. Mustafa Kahtan Al-BayatyIraqiNo ratings yet
- Cyp 450Document32 pagesCyp 450kondaveeti sreenivasulu NaiduNo ratings yet
- HyperammonemiaDocument10 pagesHyperammonemiaAsfoor gake1No ratings yet
- Lect1 - 2017Document28 pagesLect1 - 2017George MakoriNo ratings yet
- امتحان برومتريك ميكروبيولوجي 19-11-2011Document7 pagesامتحان برومتريك ميكروبيولوجي 19-11-2011Ahmed GaberNo ratings yet
- هام جداDocument93 pagesهام جداAhmed GaberNo ratings yet
- نماذج اسئلة الهيئة السعودية للتخصصات الصحية للاخصائيين والاطباء بالمختبراتDocument68 pagesنماذج اسئلة الهيئة السعودية للتخصصات الصحية للاخصائيين والاطباء بالمختبراتAhmed GaberNo ratings yet
- Non-Protein Nitrogen (NPN) CompoundsDocument34 pagesNon-Protein Nitrogen (NPN) CompoundsAhmed GaberNo ratings yet
- Liver Function Mazen 1Document24 pagesLiver Function Mazen 1Ahmed GaberNo ratings yet
- Lipids - & - Lipoproteins - Mazen 2Document31 pagesLipids - & - Lipoproteins - Mazen 2Ahmed GaberNo ratings yet
- Pancreas Function MazenDocument15 pagesPancreas Function MazenAhmed GaberNo ratings yet
- Overview of Clinical ChemistryDocument30 pagesOverview of Clinical ChemistryAhmed Gaber0% (1)
- Enzymes: M. Zaharna Clin. Chem. 2009Document32 pagesEnzymes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Enzymes: M. Zaharna Clin. Chem. 2009Document33 pagesEnzymes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Electrolytes: M. Zaharna Clin. Chem. 2009Document20 pagesElectrolytes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Electrolytes: M. Zaharna Clin. Chem. 2009Document35 pagesElectrolytes: M. Zaharna Clin. Chem. 2009Ahmed GaberNo ratings yet
- Carbohydrates - Part - 1 - MazenDocument35 pagesCarbohydrates - Part - 1 - MazenAhmed GaberNo ratings yet
- Proteins 1: M. Zaharna Clini. Chem. 2009Document50 pagesProteins 1: M. Zaharna Clini. Chem. 2009Ahmed GaberNo ratings yet
- Carbohydrates - Part - 2 - MazenDocument28 pagesCarbohydrates - Part - 2 - MazenAhmed GaberNo ratings yet
- Blood Gases Mazen 2Document16 pagesBlood Gases Mazen 2Ahmed GaberNo ratings yet
- " Chest:: by Definition Malignant Tumor of The Lung Primary Its EtiologyDocument10 pages" Chest:: by Definition Malignant Tumor of The Lung Primary Its EtiologyAhmed GaberNo ratings yet
- 1 - MCQs Classified اسئله د محمد امامDocument68 pages1 - MCQs Classified اسئله د محمد امامAhmed GaberNo ratings yet
- Proteins: M. Zaharna Ckin. Chem. 2009Document28 pagesProteins: M. Zaharna Ckin. Chem. 2009Ahmed GaberNo ratings yet
- Blood Gases, PH, and Buffer SystemDocument22 pagesBlood Gases, PH, and Buffer SystemAhmed GaberNo ratings yet
- Synthetic Approaches Towards Tubulysins and Derivatives ThereofDocument19 pagesSynthetic Approaches Towards Tubulysins and Derivatives ThereofNgô Tuấn KiệtNo ratings yet
- Worksheet 11 KeyDocument8 pagesWorksheet 11 KeyNguyễn Minh AnhNo ratings yet
- Pah - DB Eupah - 5990-4883enDocument6 pagesPah - DB Eupah - 5990-4883enridermateNo ratings yet
- Uponor Push 23b Shunt Med Wilo Pumpe MANDocument20 pagesUponor Push 23b Shunt Med Wilo Pumpe MANschritte1No ratings yet
- MSDS ChevronnDocument9 pagesMSDS ChevronnHasan UğurluNo ratings yet
- Industrial PharmacyDocument9 pagesIndustrial PharmacyMr nobodyNo ratings yet
- Oxygen Dissolved 4500Document3 pagesOxygen Dissolved 4500Sandra Vanessa Mejia SantillanNo ratings yet
- Secrets of IITDocument5 pagesSecrets of IITAnil Kumar VermaNo ratings yet
- Science q1m2Document30 pagesScience q1m2Juana Isabel B. LunaNo ratings yet
- D and F Block ElementsDocument8 pagesD and F Block ElementsPrashanth SNo ratings yet
- Bellano Digital LaminatesDocument56 pagesBellano Digital LaminatesMital DamaniNo ratings yet
- COQ Exxon-Intertek Maret 19Document1 pageCOQ Exxon-Intertek Maret 19satriasinagaNo ratings yet
- Chemical Moles & Formulae Review 2 (08.07.21)Document4 pagesChemical Moles & Formulae Review 2 (08.07.21)Micheelle JeannethNo ratings yet
- Brochure - Producedwater - Sorbwater - FOR WEBDocument12 pagesBrochure - Producedwater - Sorbwater - FOR WEBjuan vazquezNo ratings yet
- The Mole Concept: Learning CompetencyDocument14 pagesThe Mole Concept: Learning Competencylevi0417No ratings yet
- Bonding (Diamond, Graphite, Fullerene and Silicon-Dioxide)Document1 pageBonding (Diamond, Graphite, Fullerene and Silicon-Dioxide)Safe GuardNo ratings yet
- 15-TMSS-06 R.0Document13 pages15-TMSS-06 R.0wastazoheb_700349353No ratings yet
- Chemsheets As 1005 Ionisation EnergiesDocument2 pagesChemsheets As 1005 Ionisation Energiesangel ranaNo ratings yet
- Lowmain Tech ManualDocument30 pagesLowmain Tech ManualArunava BasakNo ratings yet
- Module 1 Long QuizDocument3 pagesModule 1 Long QuizJOHANNA RACHEL VILLASISNo ratings yet
- Cell Biology: Course Code: LSE-01 Assignment Code: LSE-01/TMA/2020 Maximum Marks: 100Document26 pagesCell Biology: Course Code: LSE-01 Assignment Code: LSE-01/TMA/2020 Maximum Marks: 100Rajni KumariNo ratings yet
- Repair: Re Pair in Single-Skin ConstructionDocument15 pagesRepair: Re Pair in Single-Skin ConstructionMirceaNo ratings yet
- Boiler Feed Water and Steam ChemistryDocument4 pagesBoiler Feed Water and Steam ChemistryVajid MadathilNo ratings yet
- 12th Physics Structure of Atoms & Nuclei Notes in EnglishDocument82 pages12th Physics Structure of Atoms & Nuclei Notes in EnglishAman Singh RaoNo ratings yet
- US8961680Document12 pagesUS8961680subramanian.sNo ratings yet
- Karnataka - Notified Protection Officers: (District Level)Document35 pagesKarnataka - Notified Protection Officers: (District Level)astuteNo ratings yet
- Charaterization of Liquid Solid ReactionDocument8 pagesCharaterization of Liquid Solid ReactionYuni_Arifwati_5495No ratings yet
- Pharma Manual PDFDocument24 pagesPharma Manual PDFLawrence Agada88% (8)
- Ion Exchange With Natural Zeolites: An Alternative For Water Softening?Document7 pagesIon Exchange With Natural Zeolites: An Alternative For Water Softening?Yana ElzyNo ratings yet