Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
8 viewsChap 7
Chap 7
Uploaded by
Monil KoradiaFor parallel reactions, the concentration of reactants determines product distribution. A high concentration favors higher-order reactions, a low concentration favors lower-order reactions, and concentration has no effect if reactions are the same order. Proper contacting patterns of reactants can achieve desired concentrations and control product distribution. Quantitative analysis using rate equations determines exact product distribution and reactor sizing requirements.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You might also like
- General Chemistry 2 Online: Equilibrium and Le Châtelier's PrincipleDocument12 pagesGeneral Chemistry 2 Online: Equilibrium and Le Châtelier's PrincipleirfanNo ratings yet
- A Laboratory Manual of Physical PharmaceuticsFrom EverandA Laboratory Manual of Physical PharmaceuticsRating: 2.5 out of 5 stars2.5/5 (2)
- Hot-Rolled and Cold-Finished Age-Hardening Stainless Steel Bars and ShapesDocument8 pagesHot-Rolled and Cold-Finished Age-Hardening Stainless Steel Bars and ShapesradziNo ratings yet
- D870 15Document3 pagesD870 15mithilesh100% (1)
- Design For Parallel Reactions (Compatibility Mode)Document8 pagesDesign For Parallel Reactions (Compatibility Mode)Jeetendra YadavNo ratings yet
- CRE 1 Materials - Unit 4 and 5Document69 pagesCRE 1 Materials - Unit 4 and 5Shivam SinghNo ratings yet
- Multiple ReactionDocument19 pagesMultiple ReactionHridyaAshokanNo ratings yet
- CRE Chapter 5-Design For Multiple Reactions - K192Document27 pagesCRE Chapter 5-Design For Multiple Reactions - K192Huy Huu HuynhNo ratings yet
- Design For Multiple ReactionsDocument43 pagesDesign For Multiple Reactionsmuhammad shahadat awanNo ratings yet
- Explanation For SimulationDocument5 pagesExplanation For SimulationRj JunsayNo ratings yet
- CRD 01 PDFDocument69 pagesCRD 01 PDFurwah naveedNo ratings yet
- Chemistry Unit 4 PDFDocument60 pagesChemistry Unit 4 PDFsammam mahdi samiNo ratings yet
- Chapter 7 EquilibriumDocument5 pagesChapter 7 Equilibriumapi-392847673No ratings yet
- Chemical Reactor Selection and DesignDocument36 pagesChemical Reactor Selection and Designrajadcet12100% (4)
- UNIT-3: Department of Chemical Engineering Sathyabama UniversityDocument16 pagesUNIT-3: Department of Chemical Engineering Sathyabama UniversityBT20CME033 Gautam TahilyaniNo ratings yet
- Che 416 L8Document29 pagesChe 416 L8jamil ahmedNo ratings yet
- State of EquilibriumDocument25 pagesState of EquilibriumBrent Nillas ZuñigaNo ratings yet
- CreDocument8 pagesCreBHOWMICK PATIDAR 15BCH0085No ratings yet
- Cre PDFDocument8 pagesCre PDFBHOWMICK PATIDAR 15BCH0085No ratings yet
- FullDocument33 pagesFullEja RotiKeju100% (2)
- TSFX Chem Tips PDFDocument19 pagesTSFX Chem Tips PDFIra HenryNo ratings yet
- Revision Notes On Chemical Kinetics For JEE Advanced 2024 - Free PDF DownloadDocument5 pagesRevision Notes On Chemical Kinetics For JEE Advanced 2024 - Free PDF Downloadsoulknight8 4No ratings yet
- Chap 8Document22 pagesChap 8Monil KoradiaNo ratings yet
- Chapter 6Document22 pagesChapter 6sanad.farhatNo ratings yet
- Classes Hysys 5 Reactions and ReactorsDocument7 pagesClasses Hysys 5 Reactions and ReactorsAhmed AliNo ratings yet
- KineticsDocument55 pagesKineticsSaptanshu SamalNo ratings yet
- Chemical Reactor Design and SelectionDocument36 pagesChemical Reactor Design and SelectionShaaban Ali100% (1)
- CH 1 OnlineDocument10 pagesCH 1 OnlinepalesaNo ratings yet
- Chemical KineticsDocument53 pagesChemical KineticsEuann MagtibayNo ratings yet
- Chap 1 CREDocument24 pagesChap 1 CREtuansyafiqNo ratings yet
- Chemical Equilibrium (CURRENT) - CN - STDT1BDocument1 pageChemical Equilibrium (CURRENT) - CN - STDT1BNkemzi Elias NzetengenleNo ratings yet
- Kineti CS: Things That Effect Rate of ReactionDocument5 pagesKineti CS: Things That Effect Rate of ReactionzozoxoNo ratings yet
- The Equilibrium LawDocument2 pagesThe Equilibrium Lawusulasia777No ratings yet
- EdexcelA2ChemistryRG 9781846905964 Pg8-17 WebDocument10 pagesEdexcelA2ChemistryRG 9781846905964 Pg8-17 Webmarina_shawkyNo ratings yet
- Collision TheoryDocument5 pagesCollision TheoryJenmar HemmingsNo ratings yet
- UNIT4-Chapter1-Rates Edexcel ChemistryDocument6 pagesUNIT4-Chapter1-Rates Edexcel ChemistryamanghattoraNo ratings yet
- LAS Week 2 GenChem2-Q2Document6 pagesLAS Week 2 GenChem2-Q2Drech LanadoNo ratings yet
- Experiment 2: Determination of Rate LawsDocument11 pagesExperiment 2: Determination of Rate LawsgrcmllssNo ratings yet
- Documento Traducir Reaccion PermanganatoDocument11 pagesDocumento Traducir Reaccion Permanganatoveronica RodriguezNo ratings yet
- Final Report PFRDocument12 pagesFinal Report PFRmark_ancotNo ratings yet
- Process Control Chap 8 Feedforward, Ratio ContolDocument17 pagesProcess Control Chap 8 Feedforward, Ratio ContolAishah RashiddiNo ratings yet
- Simulation of Catalytic ProcessesDocument33 pagesSimulation of Catalytic ProcessesAkhi Sofi100% (4)
- Chemical EquilibriumDocument4 pagesChemical EquilibriumDraver ZemaNo ratings yet
- 2 Design of Isothermal Ideal ReactorsDocument28 pages2 Design of Isothermal Ideal ReactorsGalata BaneNo ratings yet
- Chemical Kinetics Water Water Chemical Kinetics Water WaterDocument38 pagesChemical Kinetics Water Water Chemical Kinetics Water WateranaNo ratings yet
- Kinetics of Chemical Reactions in FoodsDocument44 pagesKinetics of Chemical Reactions in FoodsSolomon GebremariamNo ratings yet
- Principles of Chemical EquilibriumDocument17 pagesPrinciples of Chemical EquilibriumkaditasookdeoNo ratings yet
- 16 1 Rate Expression11111Document2 pages16 1 Rate Expression11111NguyenHoangMinhDucNo ratings yet
- Cre 1Document52 pagesCre 1Jayakaran PachiyappanNo ratings yet
- EquilibriumDocument4 pagesEquilibriumBikave JohnsonNo ratings yet
- Control and Pro File Setting of Reactive Distillation Column For Benzene Chloride Consecutive Reaction SystemDocument10 pagesControl and Pro File Setting of Reactive Distillation Column For Benzene Chloride Consecutive Reaction SystemHesam AhmadianNo ratings yet
- Kinetics (4.3 - Rates) Rate of Reaction - Rate of Change of Concentration With Respect To Time Measuring The Rate of A ReactionDocument5 pagesKinetics (4.3 - Rates) Rate of Reaction - Rate of Change of Concentration With Respect To Time Measuring The Rate of A ReactionureshimeNo ratings yet
- Tubular Flow Reactor Sample UiTM Lab ReportDocument20 pagesTubular Flow Reactor Sample UiTM Lab ReportNur AqilahNo ratings yet
- Reactor Design CH 8Document8 pagesReactor Design CH 8Sami WhiteNo ratings yet
- EquilibriumDocument3 pagesEquilibriumAnabella MaldonadoNo ratings yet
- Review EQUILIBRIUM REVISIONDocument51 pagesReview EQUILIBRIUM REVISIONViper PotNo ratings yet
- English WB IgcseDocument23 pagesEnglish WB Igcsetoleen playzNo ratings yet
- Investigation of the Usefulness of the PowerWorld Simulator Program: Developed by "Glover, Overbye & Sarma" in the Solution of Power System ProblemsFrom EverandInvestigation of the Usefulness of the PowerWorld Simulator Program: Developed by "Glover, Overbye & Sarma" in the Solution of Power System ProblemsNo ratings yet
- Chemical Reaction Kinetics: Concepts, Methods and Case StudiesFrom EverandChemical Reaction Kinetics: Concepts, Methods and Case StudiesNo ratings yet
- Vacuum Technology Book II Part 1 PDFDocument88 pagesVacuum Technology Book II Part 1 PDFMuhammad Maratab Ali ZiaiNo ratings yet
- Lecture #9Document33 pagesLecture #9vanshikaNo ratings yet
- (EXTRACT) Etabs Analysis Reference v18 - Friction Pendulum - Single, Double & TripleDocument13 pages(EXTRACT) Etabs Analysis Reference v18 - Friction Pendulum - Single, Double & TripleO SNo ratings yet
- Advantages of Using Ramsey Silent ChainDocument3 pagesAdvantages of Using Ramsey Silent ChainNADYANo ratings yet
- 1.4.2 Momentum 00-10Document10 pages1.4.2 Momentum 00-10Murray PhysicsNo ratings yet
- Introduction To Compressed Air SystemDocument23 pagesIntroduction To Compressed Air SystemMohd Zulhairi Mohd NoorNo ratings yet
- Case Hardening Heat TreatmentDocument15 pagesCase Hardening Heat TreatmentHazrat BelalNo ratings yet
- Duct Sealing in Large BuildingsDocument31 pagesDuct Sealing in Large BuildingsShiyamraj ThamodharanNo ratings yet
- PC160LC-8 KEPB215100 RedDocument424 pagesPC160LC-8 KEPB215100 RedRexNo ratings yet
- Position Count Description SP 30-13: Product Photo Could Vary From The Actual ProductDocument1 pagePosition Count Description SP 30-13: Product Photo Could Vary From The Actual ProducttomoNo ratings yet
- Tencord KB (E 42 4 Z B 42 h5)Document1 pageTencord KB (E 42 4 Z B 42 h5)brunizzaNo ratings yet
- Unit-3 1Document17 pagesUnit-3 1Deepak Sah100% (1)
- Earth Science 2Document32 pagesEarth Science 2Roejhen BalmacedaNo ratings yet
- Peskin & Schroeder - The Quantum SpinDocument6 pagesPeskin & Schroeder - The Quantum SpinGiggs SandyNo ratings yet
- Din 720Document28 pagesDin 720Dule JovanovicNo ratings yet
- Extended Elastic Impedance EEI Inversion PDFDocument37 pagesExtended Elastic Impedance EEI Inversion PDFsonu420No ratings yet
- Aceite Paroil Atlas Copco VariedadesDocument1 pageAceite Paroil Atlas Copco VariedadesRoberto ZevallosNo ratings yet
- Thin-Wall Pressure VesselsDocument10 pagesThin-Wall Pressure VesselsRichie Jude L. DefensorNo ratings yet
- Geodetic Equipment and BooksDocument3 pagesGeodetic Equipment and BooksJohn Kevin De CastroNo ratings yet
- PZTDocument6 pagesPZTsmjjazzab_288706263No ratings yet
- Continuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeDocument5 pagesContinuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeKasra GolbanNo ratings yet
- Preboards Exam Part III Answer Key 1Document11 pagesPreboards Exam Part III Answer Key 1Peter ian AutenticoNo ratings yet
- MACO CalculationDocument2 pagesMACO CalculationNishit SuvaNo ratings yet
- Daylight Prediction in Atrium BuildingsDocument5 pagesDaylight Prediction in Atrium BuildingsjmScriNo ratings yet
- Gasdynamics AE4140 Chapter 1: IntroductionDocument60 pagesGasdynamics AE4140 Chapter 1: IntroductionPythonraptorNo ratings yet
- Answers To Workbook U2 ALL eDocument7 pagesAnswers To Workbook U2 ALL erachellui52No ratings yet
- Molecular Dynamics Study of The Structural and Thermodynamic Properties of Sio2 Silica During Cooling Between 5000 and 300KDocument19 pagesMolecular Dynamics Study of The Structural and Thermodynamic Properties of Sio2 Silica During Cooling Between 5000 and 300KAnonymous pKuPK3zUNo ratings yet
- Use of Structural Steel in Overhead Transmission Line Towers - Code of PracticeDocument14 pagesUse of Structural Steel in Overhead Transmission Line Towers - Code of Practicegaurav singhNo ratings yet
Chap 7
Chap 7
Uploaded by
Monil Koradia0 ratings0% found this document useful (0 votes)
8 views14 pagesFor parallel reactions, the concentration of reactants determines product distribution. A high concentration favors higher-order reactions, a low concentration favors lower-order reactions, and concentration has no effect if reactions are the same order. Proper contacting patterns of reactants can achieve desired concentrations and control product distribution. Quantitative analysis using rate equations determines exact product distribution and reactor sizing requirements.
Original Description:
Original Title
chap 7
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentFor parallel reactions, the concentration of reactants determines product distribution. A high concentration favors higher-order reactions, a low concentration favors lower-order reactions, and concentration has no effect if reactions are the same order. Proper contacting patterns of reactants can achieve desired concentrations and control product distribution. Quantitative analysis using rate equations determines exact product distribution and reactor sizing requirements.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
8 views14 pagesChap 7
Chap 7
Uploaded by
Monil KoradiaFor parallel reactions, the concentration of reactants determines product distribution. A high concentration favors higher-order reactions, a low concentration favors lower-order reactions, and concentration has no effect if reactions are the same order. Proper contacting patterns of reactants can achieve desired concentrations and control product distribution. Quantitative analysis using rate equations determines exact product distribution and reactor sizing requirements.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 14
Chapter 7
Design for Parallel Reactions
Qualitative Discussion About Product
Distribution
• Now CA, is the only factor in this equation which we can adjust and
control.. (k1, k2, a1 and a2 are all constant for a specific system at a given
temperature) and we can keep CA low throughout the reactor by any of
the following means:
1. by using a mixed flow reactor,
2. maintaining high conversions,
3. increasing inerts in the feed, or decreasing the pressure in gas-phase
systems.
On the other hand,
we can keep CA high
• by using a batch or plug flow reactor,
• maintaining low conversions,
• removing inert from the feed, or increasing the pressure in gas phase
systems.
For the reactions of Eq. 1 let us see whether the concentration of
A should be kept high or low
• If a1 > a2, or the desired reaction is of higher order than the
unwanted reaction, Eq. 3 shows that a high reactant
concentration is desirable since it increases the R/S ratio. As a
result, a batch or plug flow reactor would favor formation of
product R and would require a minimum reactor size.
• If a1 < a2, or the desired reaction is of lower order than the
unwanted reaction, we need a low reactant concentration to
favor formation of R. But this would also require large mixed
flow reactor.
• If a1 = a2, or the two reactions are of the same order, Eq. 3
becomes R/S = k1/k2 = Constant Hence, product distribution
is fixed by k1/k2 , alone and is unaffected by type of reactor
used.
• We also may control product distribution by varying
k1/k2,. This can be done in two ways:
1. By changing the temperature level of operation. If the
activation energies of the two reactions are different,
k1/k2 can be made to vary.
2. By using a catalyst. One of the most important
features of a catalyst is its selectivity in depressing or
accelerating specific reactions. This may be a much
more effective way of controlling product distribution
than any of the methods discussed so far.
Summary
• For reactions in parallel, the concentration
level of reactants is the key to proper control
of product distribution. A high reactant
concentration favors the reaction of higher
order, a low concentration favors the reaction
of lower order, while the concentration level
has no effect on the product distribution for
reactions of the same order.
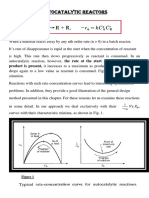
Contacting patterns for parallel reactions
• When we have two or more reactants, combinations of high and
low reactant concentrations can be obtained
1. by controlling the concentration of feed materials,
2. by having certain components in excess, and
3. by using the correct contacting pattern of reacting fluids.
• Figures 1 and 2 illustrate methods of contacting two reacting fluids
in continuous and non continuous operations that keep the
concentrations of these components both high, both low, or one
high and the other low.
• In any case, the use of the proper contacting pattern is the critical
factor in obtaining a favorable distribution of products for multiple
reactions.
Quantitative Treatment of Product
Distribution and of Reactor Size
• If rate equations are known for the individual
reactions, we can quantitatively determine product
distribution and reactor-size requirements.
• For convenience in evaluating product distribution
we introduce two terms, ѱ and Ф. First, consider
the decomposition of reactant A, and let ѱ be the
fraction of A disappearing at any instant which is
transformed into desired product R. We call this the
instantaneous fractional yield of R. Thus at any CA
You might also like
- General Chemistry 2 Online: Equilibrium and Le Châtelier's PrincipleDocument12 pagesGeneral Chemistry 2 Online: Equilibrium and Le Châtelier's PrincipleirfanNo ratings yet
- A Laboratory Manual of Physical PharmaceuticsFrom EverandA Laboratory Manual of Physical PharmaceuticsRating: 2.5 out of 5 stars2.5/5 (2)
- Hot-Rolled and Cold-Finished Age-Hardening Stainless Steel Bars and ShapesDocument8 pagesHot-Rolled and Cold-Finished Age-Hardening Stainless Steel Bars and ShapesradziNo ratings yet
- D870 15Document3 pagesD870 15mithilesh100% (1)
- Design For Parallel Reactions (Compatibility Mode)Document8 pagesDesign For Parallel Reactions (Compatibility Mode)Jeetendra YadavNo ratings yet
- CRE 1 Materials - Unit 4 and 5Document69 pagesCRE 1 Materials - Unit 4 and 5Shivam SinghNo ratings yet
- Multiple ReactionDocument19 pagesMultiple ReactionHridyaAshokanNo ratings yet
- CRE Chapter 5-Design For Multiple Reactions - K192Document27 pagesCRE Chapter 5-Design For Multiple Reactions - K192Huy Huu HuynhNo ratings yet
- Design For Multiple ReactionsDocument43 pagesDesign For Multiple Reactionsmuhammad shahadat awanNo ratings yet
- Explanation For SimulationDocument5 pagesExplanation For SimulationRj JunsayNo ratings yet
- CRD 01 PDFDocument69 pagesCRD 01 PDFurwah naveedNo ratings yet
- Chemistry Unit 4 PDFDocument60 pagesChemistry Unit 4 PDFsammam mahdi samiNo ratings yet
- Chapter 7 EquilibriumDocument5 pagesChapter 7 Equilibriumapi-392847673No ratings yet
- Chemical Reactor Selection and DesignDocument36 pagesChemical Reactor Selection and Designrajadcet12100% (4)
- UNIT-3: Department of Chemical Engineering Sathyabama UniversityDocument16 pagesUNIT-3: Department of Chemical Engineering Sathyabama UniversityBT20CME033 Gautam TahilyaniNo ratings yet
- Che 416 L8Document29 pagesChe 416 L8jamil ahmedNo ratings yet
- State of EquilibriumDocument25 pagesState of EquilibriumBrent Nillas ZuñigaNo ratings yet
- CreDocument8 pagesCreBHOWMICK PATIDAR 15BCH0085No ratings yet
- Cre PDFDocument8 pagesCre PDFBHOWMICK PATIDAR 15BCH0085No ratings yet
- FullDocument33 pagesFullEja RotiKeju100% (2)
- TSFX Chem Tips PDFDocument19 pagesTSFX Chem Tips PDFIra HenryNo ratings yet
- Revision Notes On Chemical Kinetics For JEE Advanced 2024 - Free PDF DownloadDocument5 pagesRevision Notes On Chemical Kinetics For JEE Advanced 2024 - Free PDF Downloadsoulknight8 4No ratings yet
- Chap 8Document22 pagesChap 8Monil KoradiaNo ratings yet
- Chapter 6Document22 pagesChapter 6sanad.farhatNo ratings yet
- Classes Hysys 5 Reactions and ReactorsDocument7 pagesClasses Hysys 5 Reactions and ReactorsAhmed AliNo ratings yet
- KineticsDocument55 pagesKineticsSaptanshu SamalNo ratings yet
- Chemical Reactor Design and SelectionDocument36 pagesChemical Reactor Design and SelectionShaaban Ali100% (1)
- CH 1 OnlineDocument10 pagesCH 1 OnlinepalesaNo ratings yet
- Chemical KineticsDocument53 pagesChemical KineticsEuann MagtibayNo ratings yet
- Chap 1 CREDocument24 pagesChap 1 CREtuansyafiqNo ratings yet
- Chemical Equilibrium (CURRENT) - CN - STDT1BDocument1 pageChemical Equilibrium (CURRENT) - CN - STDT1BNkemzi Elias NzetengenleNo ratings yet
- Kineti CS: Things That Effect Rate of ReactionDocument5 pagesKineti CS: Things That Effect Rate of ReactionzozoxoNo ratings yet
- The Equilibrium LawDocument2 pagesThe Equilibrium Lawusulasia777No ratings yet
- EdexcelA2ChemistryRG 9781846905964 Pg8-17 WebDocument10 pagesEdexcelA2ChemistryRG 9781846905964 Pg8-17 Webmarina_shawkyNo ratings yet
- Collision TheoryDocument5 pagesCollision TheoryJenmar HemmingsNo ratings yet
- UNIT4-Chapter1-Rates Edexcel ChemistryDocument6 pagesUNIT4-Chapter1-Rates Edexcel ChemistryamanghattoraNo ratings yet
- LAS Week 2 GenChem2-Q2Document6 pagesLAS Week 2 GenChem2-Q2Drech LanadoNo ratings yet
- Experiment 2: Determination of Rate LawsDocument11 pagesExperiment 2: Determination of Rate LawsgrcmllssNo ratings yet
- Documento Traducir Reaccion PermanganatoDocument11 pagesDocumento Traducir Reaccion Permanganatoveronica RodriguezNo ratings yet
- Final Report PFRDocument12 pagesFinal Report PFRmark_ancotNo ratings yet
- Process Control Chap 8 Feedforward, Ratio ContolDocument17 pagesProcess Control Chap 8 Feedforward, Ratio ContolAishah RashiddiNo ratings yet
- Simulation of Catalytic ProcessesDocument33 pagesSimulation of Catalytic ProcessesAkhi Sofi100% (4)
- Chemical EquilibriumDocument4 pagesChemical EquilibriumDraver ZemaNo ratings yet
- 2 Design of Isothermal Ideal ReactorsDocument28 pages2 Design of Isothermal Ideal ReactorsGalata BaneNo ratings yet
- Chemical Kinetics Water Water Chemical Kinetics Water WaterDocument38 pagesChemical Kinetics Water Water Chemical Kinetics Water WateranaNo ratings yet
- Kinetics of Chemical Reactions in FoodsDocument44 pagesKinetics of Chemical Reactions in FoodsSolomon GebremariamNo ratings yet
- Principles of Chemical EquilibriumDocument17 pagesPrinciples of Chemical EquilibriumkaditasookdeoNo ratings yet
- 16 1 Rate Expression11111Document2 pages16 1 Rate Expression11111NguyenHoangMinhDucNo ratings yet
- Cre 1Document52 pagesCre 1Jayakaran PachiyappanNo ratings yet
- EquilibriumDocument4 pagesEquilibriumBikave JohnsonNo ratings yet
- Control and Pro File Setting of Reactive Distillation Column For Benzene Chloride Consecutive Reaction SystemDocument10 pagesControl and Pro File Setting of Reactive Distillation Column For Benzene Chloride Consecutive Reaction SystemHesam AhmadianNo ratings yet
- Kinetics (4.3 - Rates) Rate of Reaction - Rate of Change of Concentration With Respect To Time Measuring The Rate of A ReactionDocument5 pagesKinetics (4.3 - Rates) Rate of Reaction - Rate of Change of Concentration With Respect To Time Measuring The Rate of A ReactionureshimeNo ratings yet
- Tubular Flow Reactor Sample UiTM Lab ReportDocument20 pagesTubular Flow Reactor Sample UiTM Lab ReportNur AqilahNo ratings yet
- Reactor Design CH 8Document8 pagesReactor Design CH 8Sami WhiteNo ratings yet
- EquilibriumDocument3 pagesEquilibriumAnabella MaldonadoNo ratings yet
- Review EQUILIBRIUM REVISIONDocument51 pagesReview EQUILIBRIUM REVISIONViper PotNo ratings yet
- English WB IgcseDocument23 pagesEnglish WB Igcsetoleen playzNo ratings yet
- Investigation of the Usefulness of the PowerWorld Simulator Program: Developed by "Glover, Overbye & Sarma" in the Solution of Power System ProblemsFrom EverandInvestigation of the Usefulness of the PowerWorld Simulator Program: Developed by "Glover, Overbye & Sarma" in the Solution of Power System ProblemsNo ratings yet
- Chemical Reaction Kinetics: Concepts, Methods and Case StudiesFrom EverandChemical Reaction Kinetics: Concepts, Methods and Case StudiesNo ratings yet
- Vacuum Technology Book II Part 1 PDFDocument88 pagesVacuum Technology Book II Part 1 PDFMuhammad Maratab Ali ZiaiNo ratings yet
- Lecture #9Document33 pagesLecture #9vanshikaNo ratings yet
- (EXTRACT) Etabs Analysis Reference v18 - Friction Pendulum - Single, Double & TripleDocument13 pages(EXTRACT) Etabs Analysis Reference v18 - Friction Pendulum - Single, Double & TripleO SNo ratings yet
- Advantages of Using Ramsey Silent ChainDocument3 pagesAdvantages of Using Ramsey Silent ChainNADYANo ratings yet
- 1.4.2 Momentum 00-10Document10 pages1.4.2 Momentum 00-10Murray PhysicsNo ratings yet
- Introduction To Compressed Air SystemDocument23 pagesIntroduction To Compressed Air SystemMohd Zulhairi Mohd NoorNo ratings yet
- Case Hardening Heat TreatmentDocument15 pagesCase Hardening Heat TreatmentHazrat BelalNo ratings yet
- Duct Sealing in Large BuildingsDocument31 pagesDuct Sealing in Large BuildingsShiyamraj ThamodharanNo ratings yet
- PC160LC-8 KEPB215100 RedDocument424 pagesPC160LC-8 KEPB215100 RedRexNo ratings yet
- Position Count Description SP 30-13: Product Photo Could Vary From The Actual ProductDocument1 pagePosition Count Description SP 30-13: Product Photo Could Vary From The Actual ProducttomoNo ratings yet
- Tencord KB (E 42 4 Z B 42 h5)Document1 pageTencord KB (E 42 4 Z B 42 h5)brunizzaNo ratings yet
- Unit-3 1Document17 pagesUnit-3 1Deepak Sah100% (1)
- Earth Science 2Document32 pagesEarth Science 2Roejhen BalmacedaNo ratings yet
- Peskin & Schroeder - The Quantum SpinDocument6 pagesPeskin & Schroeder - The Quantum SpinGiggs SandyNo ratings yet
- Din 720Document28 pagesDin 720Dule JovanovicNo ratings yet
- Extended Elastic Impedance EEI Inversion PDFDocument37 pagesExtended Elastic Impedance EEI Inversion PDFsonu420No ratings yet
- Aceite Paroil Atlas Copco VariedadesDocument1 pageAceite Paroil Atlas Copco VariedadesRoberto ZevallosNo ratings yet
- Thin-Wall Pressure VesselsDocument10 pagesThin-Wall Pressure VesselsRichie Jude L. DefensorNo ratings yet
- Geodetic Equipment and BooksDocument3 pagesGeodetic Equipment and BooksJohn Kevin De CastroNo ratings yet
- PZTDocument6 pagesPZTsmjjazzab_288706263No ratings yet
- Continuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeDocument5 pagesContinuous Modification Treatment of Polyester Fabric by Dielectric Barrier DischargeKasra GolbanNo ratings yet
- Preboards Exam Part III Answer Key 1Document11 pagesPreboards Exam Part III Answer Key 1Peter ian AutenticoNo ratings yet
- MACO CalculationDocument2 pagesMACO CalculationNishit SuvaNo ratings yet
- Daylight Prediction in Atrium BuildingsDocument5 pagesDaylight Prediction in Atrium BuildingsjmScriNo ratings yet
- Gasdynamics AE4140 Chapter 1: IntroductionDocument60 pagesGasdynamics AE4140 Chapter 1: IntroductionPythonraptorNo ratings yet
- Answers To Workbook U2 ALL eDocument7 pagesAnswers To Workbook U2 ALL erachellui52No ratings yet
- Molecular Dynamics Study of The Structural and Thermodynamic Properties of Sio2 Silica During Cooling Between 5000 and 300KDocument19 pagesMolecular Dynamics Study of The Structural and Thermodynamic Properties of Sio2 Silica During Cooling Between 5000 and 300KAnonymous pKuPK3zUNo ratings yet
- Use of Structural Steel in Overhead Transmission Line Towers - Code of PracticeDocument14 pagesUse of Structural Steel in Overhead Transmission Line Towers - Code of Practicegaurav singhNo ratings yet