Professional Documents
Culture Documents
Washing Soda
Washing Soda
Uploaded by
Arsh JindalOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Washing Soda
Washing Soda
Uploaded by
Arsh JindalCopyright:
Available Formats
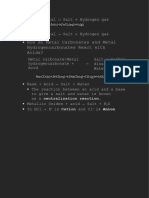
Washing soda ◦ Uses of washing soda ( Na2CO3.
10H2O )
Washing soda chemically known as sodium ◦ 1) It is used in soap, glass and paper industries .
carbonate is a white odourless powder. Washing◦ 2) It can be used as a cleansing agent for domestic purposes .
soda can be obtained from NaCl . When NaCl
reacts with ammonia , water and carbon ◦ 3) It is used in the manufacture of sodium compounds such as
dioxide ,sodium bicarbonate are obtained along borax ( a white compound which occurs as a mineral in some
with Ammonium chloride .
alkaline salt deposits ) .
NaCl + NH4 + CO2 + H2O NaHCO3 + NH4Cl ◦ 4) It is also used for removing permanent hardness of water .
Sodium bicarbonate is partially soluble in water
and hence it is precipitated as a solid . It is
filtered , dried and heated to obtain sodium
carbonate .
Bhavika Jindal
NaHCO3 2 Na2CO3 + H2O + CO2
Washing soda is produced by recrystallization of
sodium carbonate . X-D
Na2CO3 + 10H2O Na2CO3.10H2O
You might also like
- 10th Acid Base and Salt Notes 2011Document5 pages10th Acid Base and Salt Notes 2011Ashraf Husain100% (4)
- Shortcut ch2Document5 pagesShortcut ch2vemayis701No ratings yet
- Chemical CompoundsDocument17 pagesChemical CompoundsKalu ChouhanNo ratings yet
- CH 2 Topic - Salts 2Document5 pagesCH 2 Topic - Salts 2siratNo ratings yet
- Chemistry Project: Name: Azad Abdullahi Class: Ss3S Teacher: Mr. AdekunleDocument14 pagesChemistry Project: Name: Azad Abdullahi Class: Ss3S Teacher: Mr. Adekunleapi-383198550% (2)
- Chemicals From Common Salt 2Document24 pagesChemicals From Common Salt 2KhushiNo ratings yet
- ABS - Important ChemicalsDocument20 pagesABS - Important ChemicalsAnoushka NathNo ratings yet
- Salts-1Document7 pagesSalts-1The Good BoysNo ratings yet
- Washing Soda IndustryDocument15 pagesWashing Soda IndustryHasnain Ali0% (1)
- Sodiumcarbonate 180826152936Document31 pagesSodiumcarbonate 180826152936Aliha AzmatNo ratings yet
- Ch-2 Part-3Document4 pagesCh-2 Part-3Kartik BhardwajNo ratings yet
- 1928 ADocument5 pages1928 AMarvel and D.C. EducationNo ratings yet
- SaltsDocument22 pagesSaltssaanvi katyalNo ratings yet
- Sodium Carbonate 861: Occurrence and UsesDocument2 pagesSodium Carbonate 861: Occurrence and UsesAHMEDNo ratings yet
- Salts PDFDocument7 pagesSalts PDFpiyushNo ratings yet
- 10 Chemistry ABS 6Document2 pages10 Chemistry ABS 6Aryan GuptaNo ratings yet
- Acids Bases and SaltsDocument4 pagesAcids Bases and SaltsMandeep SinghNo ratings yet
- Chemistry Grade 10 On SaltsDocument11 pagesChemistry Grade 10 On SaltsKolade Fatai OpeyemiNo ratings yet
- S Block NotesDocument7 pagesS Block NotesSiddharth SangaiNo ratings yet
- Washing Soda-1Document25 pagesWashing Soda-1Muhammad RamzanNo ratings yet
- Solvay Process PDFDocument8 pagesSolvay Process PDFMuhammad RafiqueNo ratings yet
- S.3 Chemistry MR SsemugoomaDocument9 pagesS.3 Chemistry MR SsemugoomalionlioneenjohnsmithNo ratings yet
- Chapter-2 Acids, Bases and Salts: PropertiesDocument2 pagesChapter-2 Acids, Bases and Salts: PropertiesAkASHNo ratings yet
- Solvay Process: Chapter 16 - Chemical IndustriesDocument12 pagesSolvay Process: Chapter 16 - Chemical IndustriesMuneeba ArifNo ratings yet
- Nitric Acid (SUMMARY CHEMISTRY CHAPTER)Document2 pagesNitric Acid (SUMMARY CHEMISTRY CHAPTER)the lillyNo ratings yet
- Industri Klor AlkaliDocument24 pagesIndustri Klor AlkaliUtopia SasaNo ratings yet
- Nitric AcidDocument9 pagesNitric Acidaditya varteNo ratings yet
- CP-XVII (Soda Ash & Caustic Soda)Document12 pagesCP-XVII (Soda Ash & Caustic Soda)Usman AliNo ratings yet
- D BlockDocument20 pagesD BlockRaju SinghNo ratings yet
- Sodium CarbonateDocument6 pagesSodium CarbonateLihini NimsaraNo ratings yet
- Acids 1Document4 pagesAcids 1Syed AyaanNo ratings yet
- S Block Lecture 2 PDFDocument9 pagesS Block Lecture 2 PDFRobiul AlomNo ratings yet
- UNIT I - Water Treatment: (Soluble Sodium Stearate) (Insoluble Ca-Stearate) SoapDocument25 pagesUNIT I - Water Treatment: (Soluble Sodium Stearate) (Insoluble Ca-Stearate) Soap52 Shagun ChaudhariNo ratings yet
- ICSE Class 10 Chemistry Chapter 10 - Nitric Acid Revision NotesDocument2 pagesICSE Class 10 Chemistry Chapter 10 - Nitric Acid Revision NotesShaunak OrigamiNo ratings yet
- Solvay Process of Soda Ash ManufactureDocument16 pagesSolvay Process of Soda Ash ManufactureChristine FernandezNo ratings yet
- Sodium Bicarbonate & Sodium CarbonateDocument2 pagesSodium Bicarbonate & Sodium CarbonatePablo Inda HuertaNo ratings yet
- Chemicals Obtained From SaltsDocument15 pagesChemicals Obtained From SaltsParas AroraNo ratings yet
- Notes Acids Bases and SaltsDocument8 pagesNotes Acids Bases and SaltsWouldn't you like to knowNo ratings yet
- Acid, Bases and Salts - NotesDocument5 pagesAcid, Bases and Salts - NotespritiNo ratings yet
- Sulphuric Acid (SUMMARY CHEMISTRY CHAPTER)Document3 pagesSulphuric Acid (SUMMARY CHEMISTRY CHAPTER)the lillyNo ratings yet
- Grade 10 Acids, Bases, and Salts: Reaction With MetalsDocument3 pagesGrade 10 Acids, Bases, and Salts: Reaction With Metals22550No ratings yet
- S&P Block PDFDocument1 pageS&P Block PDFvrtbhgmngfNo ratings yet
- MuốiCacbonateDocument3 pagesMuốiCacbonatenhyd115692No ratings yet
- Softening FinalDocument23 pagesSoftening FinalSonali Jahagirdar100% (1)
- Sodium Hydrogen Carbonate Sodium Hydrogen Carbonate and Sodium Carbonate and Sodium CarbonateDocument2 pagesSodium Hydrogen Carbonate Sodium Hydrogen Carbonate and Sodium Carbonate and Sodium CarbonateRachael LeeNo ratings yet
- 10th SCIENCE (English Medium) Must DoDocument63 pages10th SCIENCE (English Medium) Must Doanshu26stNo ratings yet
- Acids Bases and SaltsDocument3 pagesAcids Bases and SaltsNishant KumarNo ratings yet
- PPT From Other SourcesDocument33 pagesPPT From Other SourcesHarshit GoelNo ratings yet
- Acids, Bases and Salts Notes - Part 4Document7 pagesAcids, Bases and Salts Notes - Part 4Dhyan ShahNo ratings yet
- Sodium Carbonate: NA CODocument44 pagesSodium Carbonate: NA COMg HNo ratings yet
- Industrial Inorganic ChemistryDocument17 pagesIndustrial Inorganic ChemistryMUHAMMAD NABEEL ARIFNo ratings yet
- Sodium CarbonateDocument1 pageSodium CarbonateAmr hishamNo ratings yet
- Production Technology of Cloro-Alkali IndustriesDocument71 pagesProduction Technology of Cloro-Alkali IndustriesBereket Tadesse100% (1)
- 7 Sodium CarbonateDocument2 pages7 Sodium CarbonateManit ShahNo ratings yet
- Introduction of Production of Caustic SodaDocument14 pagesIntroduction of Production of Caustic SodaAlia Khan100% (2)
- Production: Sodium Bicarbonate or Sodium Hydrogen Carbonate Is TheDocument1 pageProduction: Sodium Bicarbonate or Sodium Hydrogen Carbonate Is TheSandeep PalNo ratings yet
- The Leblanc and Solvay ProcessDocument4 pagesThe Leblanc and Solvay ProcessScribdTranslationsNo ratings yet