Professional Documents
Culture Documents
CNS Pathogens-Jesse Wackerbarth
CNS Pathogens-Jesse Wackerbarth
Uploaded by
Microposter0 ratings0% found this document useful (0 votes)
119 views1 pageHerpes Simplex Encephalitis (HSE) is a rare and dangerous brain infection caused by the Herpes simplex virus-1. It causes severe inflammation and damage to brain tissue, particularly in the temporal lobes. Left untreated, 70% of cases will result in death. Modern diagnosis involves detecting the viral genome in cerebrospinal fluid. Treatment involves high doses of the antiviral drug acyclovir. Streptococcus pneumoniae is a common cause of bacterial meningitis and can have serious complications such as brain edema, hydrocephalus, and cerebral infarctions. It is spread through respiratory droplets and evades the immune system through its thick polysaccharide capsule.
Original Description:
Rough draft--still some citations and captions to be added.
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentHerpes Simplex Encephalitis (HSE) is a rare and dangerous brain infection caused by the Herpes simplex virus-1. It causes severe inflammation and damage to brain tissue, particularly in the temporal lobes. Left untreated, 70% of cases will result in death. Modern diagnosis involves detecting the viral genome in cerebrospinal fluid. Treatment involves high doses of the antiviral drug acyclovir. Streptococcus pneumoniae is a common cause of bacterial meningitis and can have serious complications such as brain edema, hydrocephalus, and cerebral infarctions. It is spread through respiratory droplets and evades the immune system through its thick polysaccharide capsule.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
119 views1 pageCNS Pathogens-Jesse Wackerbarth
CNS Pathogens-Jesse Wackerbarth
Uploaded by
MicroposterHerpes Simplex Encephalitis (HSE) is a rare and dangerous brain infection caused by the Herpes simplex virus-1. It causes severe inflammation and damage to brain tissue, particularly in the temporal lobes. Left untreated, 70% of cases will result in death. Modern diagnosis involves detecting the viral genome in cerebrospinal fluid. Treatment involves high doses of the antiviral drug acyclovir. Streptococcus pneumoniae is a common cause of bacterial meningitis and can have serious complications such as brain edema, hydrocephalus, and cerebral infarctions. It is spread through respiratory droplets and evades the immune system through its thick polysaccharide capsule.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 1
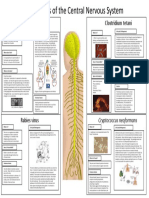
Herpes Simplex Encephalitis (HSE) Streptococcus pneumoniae: Bacterial Meningitis
Streptococcus pneumoniae is a gram positive, diplococci shaped bacteria of the phylum
HSE is a rare infection of the brain by the Herpes simplex virus-1 (HSV-1), the causative pathogen of firmicutes. It is an extracellular pathogen and one of the most prevalent and serious causes
common orofacial cold sores. Infection causes severe necrotizing encephalitis with severe of bacterial meningitis in humans, proving fatal for 30% of patients and causing long-term
neuroinflammation and swelling of the brain (particularly the temporal lobes, where small hemorrhages neural sequelae in around 40% of survivors. Streptococcus pneumoniae (or pneumococcus)
may occur), leading to the development of classic encephalitic symptoms including confusion, altered
mental status, personality changes, fever, and potentially seizures. The condition is extremely dangerous Pathogenic is part of the normal flora observed in the human upper respiratory tract, but can
opportunistically cause disease under the right conditions, particularly in response to
to the fragile CNS and 70% untreated cases will be fatal; of those treated a third will die and only 20% immune suppression and with the assistance of several virulence factors. It can also be
will recover without long term neuro-cognitive damage. Modern diagnosis’s are typically made through
PCR detection of the viral genome in the CSF and treated with high dosages of Acyclovir—an antiviral
Infections of spread through respiratory droplets or direct contact leading to colonization of the
nasopharynx as the first stage of development, initially binding to specific carbohydrate
that selectively inhibits viral DNA polymerase. signatures on host epithelial cells. Pnumocuccus must then invade the intravascular space,
HSV-1 is a member of the Herpesviridae and is a relatively large, double stranded DNA, enveloped virus.
the CNS which requires polymeric immunoglobulin receptor (pIgR) on the human cell surface and
CbpA on the pneumococcus. Hyaluronate lyase released by the bacteria also has been
It is spread primarily by direct contact with a localized infected areas (classically cold sores) though there Major CNS complications secondary to acute bacterial meningitis. (A) Brain
shown tooedema.
degrade the extracellular matrix of connective tissues and allow greater
Jesse Wackerbarth (B) Hydrocephalus. (C) Cerebral vasculitis with multiple cerebral infarctions. (D) Sinus
can still be some shedding of viral particles in the absence of a symptomatic manifestation. HSV gains infiltration.
thrombosis with venous infarction and mild cerebral haemorrhage (black arrow).
cellular entry by envelope fusion with target membranes based on glycolipid-recetor interactions. Once
inside, HSV-1 capsid travels to the cell nucleus, where it injects its genome through a portal generated by Once it has gained access to the bloodstream pneumocuccus evades the immune system
UL6 proteins. Notably, the virion host shutoff protein (VHS or UL41) inhibits host protein synthesis and Our central nervous system is the most important and complex system in the human with its thick polysaccharide capsule, a major virulence factor that cloaks the bacteria’s
degrades host mRNA. During the lytic cycle, herpes virus protein genes, classified immediate-early, early, body, but also one of the most fragile. The billions of Neurons that constitute the CNS, antigenic surfaces and provides a strong anti-phagocytic advantage. Additionally, capsule
and late are transcribed then translated and the complete virus is assembled in the nucleus; eventually, allowing us to think, move, breath, and live, are special; unlike most other cells in the provides protection from the attacks of complement and the production of antibodies.
through a complex pathway of nuclear budding, replicated virions are excytosed from the host cell. body, they cannot divide or be effectively repaired, the neurons we have are Pneumococcal surface proteins (Psp) A and C also perform sheltering functions against the
irreplaceable and thus damage to the CNS is much more devastating than in other binding of c3b and the membrane attack complex. Production of the toxin Pneumolysin,
HSV-1 also has the potential for latency, in which viral proteins of the lytic cycle are not produced and the areas of the body. While the classic immune response is essential and effective in most further inhibits the complement response by binding to the fc region of IGg, and generating
virus lies dormant particularly in the sensory neural ganglia. It is currently speculated that from this of the body, its veracity often leads to extensive localized tissue damage from our own a false classic pathway response.
neuro-infective capability, through unknown mechanisms and activations, HSV-1 virus gains entry to the immune cells. For most physiological systems this is necessary and generally reparable
peripheral neural system and migrates through the peripheral axons to infect the CNS (via retrograde collateral damage, but for the CNS it can be devastating and irreversible. Furthermore, Pneumococcal meningitis typically requires a high bacterial load in the bloodstream prior
axonal flow), thereby avoiding the formidable BB barrier. It remains unclear precisely how and where encasement within the rigid skull and spinal column leaves very little room for to CNS infiltration. The exact site of entry into the CSF remains unclear and highly debated.
this infiltration occurs, though current research has implicated the olfactory nerve as a likely candidate. inflammation without dangerous consequences. Thus it is necessary that the CNS be The general strategy involves first attaching to epithelial cells at several glycoconjugates.
Nevertheless, once accessed, the CNS is particularly susceptible to HSV infection because the immunologically privileged, distinct from the peripheral immune system and more They then activate the host epithelial cells to increase the expression of surface platelet-
intraneuronal spread is believed to shelter virus from host defense mechanisms. delicate in its responses. Immune privileged areas are characterized partly by a greater activating factor (PAF) receptor, which binds to phosphorylcholine in the bacterial cell wall.
tolerance to potential antigen, such as transplanted tissues or foreign organisms, but When bound, PAF receptors are coded to endocytose, in this case bringing along the
Pathologically, HSV infected cells can balloon in size, degrading the plasma membrane and nuclear also by how they mount a response when necessary; thus the CNS is not immune- attached pneumococcus into the interior of the cell. Though some will perish within the
structure into multi nucleated large cells. The virus elicits a strong immune response, engaging first the deficient, but highly immune-specialized. host cell, the bacteria (though not an intercellular pathogen) can travel through the
microglia, which up-regulate their activity and expression of antigen presenting MHC proteins, and leads epithelial cell, and emerge to infect the CNS.
to increased infiltration of granulocytes and t lymphocytes to the site of infection. HSV-1 has been The CNS is separated from blood stream (and much of the generalized immune
demonstrated to productively infect both neurons and astrocytes, but seems to have little productive system) by the blood-brain barrier, a system of electrically resistant tight junctions Once inside the CNS, microglia respond to infection but are even more inept in their
infectious capability in microglia. Microglia however have been show to induce apoptosis and release linking the epithelial cells that line the capillaries providing the brain’s blood supply. phagocytic activity than the peripheral immune cells. Interestingly, phase variation, in
large quantities of neurotoxic cytokines when infected even nonproductively. Thus damage to the CNS This cellular barrier allows gas diffusion and nutrient transfer through transport, but which CNS invasive pneumococcus express higher levels of teichoic acid and cell wall
tissue is thought to be partly from the infectious activities of the virus, but perhaps more critically from generally prevents the migration of large particles, both pathogenic and immune, from proteins relative to capsule, seems to heighten the CNS response. Typical antigenic
the wide spectrum of cytotoxic compounds and acute inflammation elicited by the immune response. the blood stream into the cerebral spinal fluid (CSF), thereby isolating and protecting recognition of PAMP regions and the toxin Pneumolysin leads to a strong immune response,
This is evidenced in the prolonged activation of microglia for up to 12 months after treatment with the CNS environment; externally, it is protected by the covering of leptomeninges and consisting first in the activation of microglia, releasing damaging inflammatory and
antivirals and resolution. The latency and reactivation characteristics of herpes simplex in other regions skull. The permeability of the BB barrier is thought to be regulated by the activity of cytotoxic cytokines, and second, in the destructive recruitment of peripheral immune cells.
of the body is not typically observed in HSE, as the retrograde axonal migration of the virus appears adjacent CNS cells called astrocytes. Similarly, the blood-CSF barrier provides a Both contribute to swelling and neural cell destruction that leads to symptomatic disease
extremely rare phenomena and is not linked to prolonged infection, as only 10% of those who develop protective layer in the choroid plexuses and the arachnoid membrane, between the and potentially death; the bacteria itself, lacking the capacity for neural intracellular
HSE report having a history of recurrent cold sores or other HSV manifestations. dura and the subarachnoid fluid. Thus, pathogens that infect the CNS must have some invasion or serious toxic production does little real CNS damage.
mechanism for avoiding or overcoming these formidable barriers. While the two
immune systems were once assumed to be almost entirely separate, new research has
Cerebral Malaria: Plasmodium falciparum made it increasingly clear that their interactions are numerous and complex, though Mycobacterium tuberculosis: CNS Tuberculosis
the CNS system retains a moderate degree of autonomy.
Cerebral malaria (CM) is a serious and life-threatening complication of malarial disease that affects Mycobacterium tuberculosis is an aerobic, acid-fast gram positive bacilli, characterized
more than a million lives annually. Infection is primarily caused by the protozoan parasite by it slow growth and waxy cell wall with high lipid content (particularly mycolic acid). It
Microglia are the CNS macrophages and the workhorse of the localized immune
Plasmodium falciparum, which is carried and transmitted to humans by the female Anopheles is the causative agent of tuberculosis, a primarily respiratory disease affecting nearly a
response. In the absence of pathogen they play a mostly neuro-supportive role,
mosquito. The initial development of malaria involves a complex parasitic life cycle, in which third of the world’s population, which, in around 1% of cases, can progress to a rare, high
scanning for and removing damaged tissues and plaques, but in times of infection they
reproduction occurs in the liver—avoiding the attention of the immune response—and the onset of mortality infection of the CNS. Left untreated, CNS tuberculosis is invariably fatal. The
are activated and act as specialized CNS immune soldiers. Microglia have many
clinical malarial disease is marked by the emergence of parasite into the blood stream, where they onset of neurological symptoms progresses similarly to most CNS infections, beginning
functions, including the classics: non-specific antigen recognition, phagocytic and
Section of brain showing blood preferentially
vessels infect red blood cells and cause the characteristic fever and chills. Malaria is an with mild complaints like headache, fever, or dizziness and progressing to severe
cytotoxic activity, cytokine production, as well as antigen presentation and t cell
blocked with developing P. falciparum
enormous global health challenge, infecting over 250 million people annually though only 1 to 2% neurocognitive disturbances, altered mental status and seizures typical of CNS
parasites (see arrows) (RPH). will develop a neurological manifestation called cerebral malaria (CM), the majority of which will be activation in substantial infections; great plasticity and sensitivity is necessary due to
inflammation. Thus its rarity and lack of good diagnostic techniques, makes early
their isolated, fragile environment—for only in cases of extreme infection are
children. CM is characterized by the development of neurological symptoms accompanying classic identification of CNS tuberculosis daunting problem.
peripheral phagocytes recruited through a degraded BB barrier. Recent research has
malaria infection. Victims may become delirious, confused, altered, or dizzy and can progress to
indentified both the expression of MHC class I and II, as well as communication with
seizures, coma, and death. Mycobacterium tuberculosis (MTB) first infects the human host through inhalation of
peripheral t lymphocytes, yet many of the mechanisms and consequences of this
respiratory droplets from an individual with an active TB infection. MTB is a facultative
interaction remain unclear.
The neuropathology of CM still not very well understood, however researchers have demonstrated intercellular pathogen that preferentially infects the alveolar macrophages through a
that it begins with high levels of parasitically infected erythrocytes (red blood cells) in the blood
variety of receptors inducing phagocytosis. Once inside, M. tuberculosis’s hydrophobic
stream. Infection of red blood cells may enhance the expression of P. falciparum erythrocyte . cell wall and other virulence hijack the phagosome and proliferate within the immune
membrane protein (PfEMP-1), which binds to ligands on endothelial cells, such as ICAM-1 or E- cells. Classically, pulmonary MTB infection produces a massive inflammatory response
selectin. As masses of infected erythrocytes adhere to the deep microvasculature serving the CNS, and the charteristic formation of granulomas; as the infection progresses low levels of
normal flux is occluded and the CNS interior may become progressively stressed and hypoxic, MTB have the capacity to spread through the blood stream and lymphatic system, and
contributing to the development of neurological symptoms and eventually coma. It has also been occasionally colonize areas such as the CNS.
implicated that a malarial toxin may induce the release of cytokines by macrophages, which leads to
the uncontrolled production and accumulation of toxic nitric oxide in the CNS. It has been speculated that MTB migrates through protective epithelial cells
independently, or within infected macrophages; however, recent research on animal
The accumulation of infected blood cells brings a rapid host peripheral immune response, in the models has indicated that MTB may not gain access to the CNS through infiltration of the
form of T lymphocytes and monocytes—further crowding the already occluded capillaries. blood-brain barrier, as is typical of bacterial CNS invasions. Instead MTB can gain access
Interestingly, the CNS immune system responds as well, probably from both environmental stress to the subarachnoid space through the rupture of adjacent parenchymal tubercle or a
Intact erythrocytes surrounding a capillary in the cerebral cortex. Endothelial
and an influx of cytokine signaling, leading to the activation of microglia. Activated microglia cell layer appears to have disintegrated (arrow) caseating vascular focus, thereby bypassing the barrier defense and gaining entry to the
contribute to even greater cytokine production from both sides, especially TNF-α, and result in the vulnerable CNS.
degradation of the epithelial blood brain barrier, with some observed migration of microglia cells.
As the epithelial layer weakens, micro hemorrhaging can occur into the CNS bringing with it Once inside the CNS, M. tuberculosis effectively and productively infects microglia cells
infected erythrocytes and elements of the immune response. Immune activation, as well as some due to their mechanistic similarities to macrophages. The facilitate uptake via a variety
cytotoxic production by the parasite, damages the astrocytes and decreases regulatory control, of receptors, mainly the CD14 receptor when not nonopsonized. Rapid cytokine release,
stressing the CNS neural environment. Severe, late stage cases can lead to the formation of ring like Murine model of CNS tuberculosis. (A) Coronary section at the level of particularly
the caudal of TNF-α, leads to inflammation and increased permeability of the BB
liaisons on the brain. barrier showing
diencephalon, with multifocal nonsuppurative encephalitis. (B) Cornu ammonis and the rapid recruitment of destructive peripheral immune cells, classically
forming
mild perivascular lymphocytic and histiocytic infiltration, with microgliosis disruptive tubercular granulomas throughout different areas of the CNS
and reactive
Though fatal in 30 to 40% of cases even when treated effectively with anti-malarial drugs, survivors astroglia. (C) Dorsal third ventricle, choroid plexus, and subependymal infection.
areas expanded The immune response against intercellular pathogens and with recruited
by lymphocytic, plasmacytic, and histiocytic infiltration, with subependymal microgliosis
of CM have a relatively low risk of long term neurological impairments. Since infect does not and reactive astroglia. lymphocytes is exceptionally destructive and feeds back to an even greater
include a large scale immune response within the CNS or the recruitment of the often destructive inflammatory response. Depending on the area of entry MTB can cause either
peripheral immune cells, only about 10% of cases experience long-term symptoms or deficits. Semi-thin section of capillary in brainstem. Enlarged perivascular space (*) containing leukocytes in close encephalitis or meningitis, and can even form brain abscesses—all of these life-
vicinity to the vessel. Lymphocytes (arrows) and monocyte (arrowhead) sequestered to the endothelial wall. threatening conditions contribute the severe neurological symptoms and the high
You might also like
- RCL Pre-Employment Medical Examination Form B Revised 2015-03Document2 pagesRCL Pre-Employment Medical Examination Form B Revised 2015-03Ahmad Shodiq100% (1)
- Your 5 Moments For Hand Hygiene PosterDocument1 pageYour 5 Moments For Hand Hygiene PosterAniruddha Bagchi100% (1)
- Rubi Li v. Sps. SolimanDocument1 pageRubi Li v. Sps. SolimanJennifer OceñaNo ratings yet
- Jesse Wackerbarth - CNS Pathogens RevisedDocument1 pageJesse Wackerbarth - CNS Pathogens RevisedMicroposterNo ratings yet
- Pathogenesis Infection of CnsDocument4 pagesPathogenesis Infection of CnshadiNo ratings yet
- Ppat 1010234hhDocument9 pagesPpat 1010234hhChairulanisaNo ratings yet
- Parasites: MC 3 LEC-Microbiology and Parasitology LectureDocument4 pagesParasites: MC 3 LEC-Microbiology and Parasitology LectureJamie Angelo PerezNo ratings yet
- Activity IiDocument5 pagesActivity IiYVONNE PEARL BALIQUIDNo ratings yet
- Clinpharm Notes 4 TopicsDocument8 pagesClinpharm Notes 4 TopicsALESANDRA DAWN PAYOTNo ratings yet
- Emerging and Less Common Viral Encephalitides - Chapter 91Document34 pagesEmerging and Less Common Viral Encephalitides - Chapter 91Victro ChongNo ratings yet
- Vaccines 09 00209 v2Document16 pagesVaccines 09 00209 v2Rin ChanNo ratings yet
- Viral Encephalitis: A Clinician's Guide: ReviewDocument18 pagesViral Encephalitis: A Clinician's Guide: ReviewRandy UlloaNo ratings yet
- Neuro B2.1 Viral Infections of The CNSDocument10 pagesNeuro B2.1 Viral Infections of The CNSKendel Ann KempisNo ratings yet
- 2021 - Acute Neurologic Manifestations of Respiratory VirusesDocument17 pages2021 - Acute Neurologic Manifestations of Respiratory VirusesOlga Manco GuzmánNo ratings yet
- Contagious Diseases in PHDocument10 pagesContagious Diseases in PHJEnLipataNo ratings yet
- MICP (Autosaved) DONEDocument7 pagesMICP (Autosaved) DONEEdna ChanNo ratings yet
- Problem 3.01 Nervous System Study Guide 3Document2 pagesProblem 3.01 Nervous System Study Guide 3Monish NaiduNo ratings yet
- 724011.knjiga Respiratory Infections Upper RTInfections Chapter 2 2013Document13 pages724011.knjiga Respiratory Infections Upper RTInfections Chapter 2 2013Christabella Natalia WijayaNo ratings yet
- Streptococcus PneumoniaDocument2 pagesStreptococcus PneumoniaElishah CaprichoNo ratings yet
- CNS InfectionsDocument92 pagesCNS InfectionsWaaqoo Guutuu Waaqoo GuutuuNo ratings yet
- Central Nervous System InfectionsDocument33 pagesCentral Nervous System InfectionsJENSEL CLOUIE C. REGLOSNo ratings yet
- Bacterial Meningitis and Other Nonviral Infections of The Nervous SystemDocument13 pagesBacterial Meningitis and Other Nonviral Infections of The Nervous Systemgeancarla mendozaNo ratings yet
- 3 s2.0 B0122268709015719 MainDocument6 pages3 s2.0 B0122268709015719 MainasakingofhNo ratings yet
- Neurologic InfectionsDocument6 pagesNeurologic InfectionsHazel ZullaNo ratings yet
- 12 Cns Infection 2 LecturesDocument18 pages12 Cns Infection 2 LecturesZain AlAbideen AlTaeeNo ratings yet
- Guide Clinical PDFDocument19 pagesGuide Clinical PDFAbd Halim Gazali HNo ratings yet
- Atb Meningitis Coid 2018Document12 pagesAtb Meningitis Coid 2018Danny JacobusNo ratings yet
- Cshperspectmed BAC A012393Document14 pagesCshperspectmed BAC A012393RaffaharianggaraNo ratings yet
- CNS - Infections F2022 OVER VIEWDocument18 pagesCNS - Infections F2022 OVER VIEWMadison MillwoodNo ratings yet
- CHAPTER 127 MENINGOCOCCAL INFECTIONS SummaryDocument3 pagesCHAPTER 127 MENINGOCOCCAL INFECTIONS Summaryapi-3704562No ratings yet
- Jurnal Rabies 1Document5 pagesJurnal Rabies 1rahel imeldaNo ratings yet
- Bio PosterDocument1 pageBio PosterRyan LeeNo ratings yet
- Clinical MicrobiologyDocument16 pagesClinical MicrobiologyArthur YanezNo ratings yet
- Diagnostic Workup For ARDS Patients: ReviewDocument12 pagesDiagnostic Workup For ARDS Patients: ReviewwandapandabebeNo ratings yet
- J Tim 2015 12 012Document9 pagesJ Tim 2015 12 012Rin ChanNo ratings yet
- Managing Meningoencephalitis in Indian ICU: Neurocritical CareDocument5 pagesManaging Meningoencephalitis in Indian ICU: Neurocritical CareerikafebriyanarNo ratings yet
- Infectious Neuropathies. 2023Document26 pagesInfectious Neuropathies. 2023Arbey Aponte PuertoNo ratings yet
- Deniz Ot 2012Document16 pagesDeniz Ot 2012IskvcNo ratings yet
- Role of Damps in Respiratory Virus-Induced Acute Respiratory Distress Syndrome - With A Preliminary Reference To Sars-Cov-2 PneumoniaDocument20 pagesRole of Damps in Respiratory Virus-Induced Acute Respiratory Distress Syndrome - With A Preliminary Reference To Sars-Cov-2 PneumoniamacelobpachecoNo ratings yet
- Acute Neurological InfectionsDocument6 pagesAcute Neurological Infectionsramon.camara.03No ratings yet
- Disorders of Respiratory Function - Infections and Neoplasms - 2020Document17 pagesDisorders of Respiratory Function - Infections and Neoplasms - 2020Cres Padua QuinzonNo ratings yet
- Nihms 1664293Document21 pagesNihms 1664293MARIA MONTSERRAT SOMOZA MONCADANo ratings yet
- Pathogenesis and Pathophysiology of Bacterial Infections of The CNSDocument16 pagesPathogenesis and Pathophysiology of Bacterial Infections of The CNSSerque777No ratings yet
- InfluenzaDocument54 pagesInfluenzaakhmedovg16No ratings yet
- 1-S2.0-0002934388904561-MainDocument4 pages1-S2.0-0002934388904561-MainI Made AryanaNo ratings yet
- Invasive Cryptococcal Meningitis Presenting As A Skull Base Mass in An Immunocompetent Host: A Case ReportDocument5 pagesInvasive Cryptococcal Meningitis Presenting As A Skull Base Mass in An Immunocompetent Host: A Case ReportAsep RiswandiNo ratings yet
- The Neurological ManifestationsDocument13 pagesThe Neurological ManifestationsandreaNo ratings yet
- CNS Fungal InfectionDocument12 pagesCNS Fungal InfectionMuhammad Yusuf HanifNo ratings yet
- Streptococcal Skin InfectionsssDocument6 pagesStreptococcal Skin InfectionsssteenaNo ratings yet
- NCM112 LP2 TransesDocument9 pagesNCM112 LP2 TransesChristine CalleyNo ratings yet
- Central Nervous System Infections: Gonzalo B. Roman JR.,MD.,FPSPDocument72 pagesCentral Nervous System Infections: Gonzalo B. Roman JR.,MD.,FPSPBenjamin PrabhuNo ratings yet
- Approach To Neurologic InfectionsDocument18 pagesApproach To Neurologic InfectionsHabib G. Moutran Barroso100% (1)
- Microbial Diseases of The Nervous SystemDocument7 pagesMicrobial Diseases of The Nervous SystemAnaNo ratings yet
- Roos, Karen L. Greenlee, John E. - Meningitis and EncephalitisDocument14 pagesRoos, Karen L. Greenlee, John E. - Meningitis and EncephalitisNadila Nur PratiwiNo ratings yet
- تَـلـخـيـص شَـابـتـر ٢٢?Document14 pagesتَـلـخـيـص شَـابـتـر ٢٢?سلطان محمد فوزي سلمانNo ratings yet
- 12infectious DiseasesDocument6 pages12infectious DiseasesDheepak ARNo ratings yet
- Natural History of DiseaseDocument11 pagesNatural History of DiseaseScribdTranslationsNo ratings yet
- Viral CNS InfectionDocument43 pagesViral CNS Infectionissrafil mussaNo ratings yet
- Leocadio CD Outline RevisedDocument15 pagesLeocadio CD Outline RevisedJingle Domingo CanonizadoNo ratings yet
- Host-Pathogen Interactions in Bacterial MeningitisDocument25 pagesHost-Pathogen Interactions in Bacterial MeningitisEugen TarnovschiNo ratings yet
- Neuroinfections Presentation Diagnosis and Treatment of Meningitis and EncephalitisDocument10 pagesNeuroinfections Presentation Diagnosis and Treatment of Meningitis and EncephalitisZarick SaenzNo ratings yet
- Infections of The Liver Revised - Nick GriffinDocument1 pageInfections of The Liver Revised - Nick GriffinMicroposterNo ratings yet
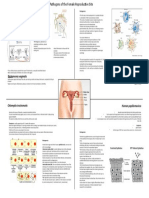
- Pathogens of The Female Reproductive Site, Erika HuertaDocument1 pagePathogens of The Female Reproductive Site, Erika HuertaMicroposterNo ratings yet
- Pathogens of The Vagina-CitationsDocument1 pagePathogens of The Vagina-CitationsMicroposterNo ratings yet
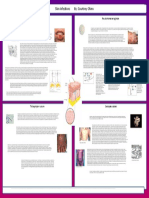
- Eye Infections by Allison BakerDocument1 pageEye Infections by Allison BakerMicroposterNo ratings yet
- Jesse Wackerbarth - CNS Pathogens RevisedDocument1 pageJesse Wackerbarth - CNS Pathogens RevisedMicroposterNo ratings yet
- Pathogens in The Lungs-Jeffrey DelgadilloDocument1 pagePathogens in The Lungs-Jeffrey DelgadilloMicroposterNo ratings yet
- Savannah Whitington: Your Brain On DrugsDocument1 pageSavannah Whitington: Your Brain On DrugsMicroposterNo ratings yet
- Pathogens of The Vagina-Annie Espinosa - This Is The Revised VersionDocument1 pagePathogens of The Vagina-Annie Espinosa - This Is The Revised VersionMicroposterNo ratings yet
- Meghan Cule-Pathogens of The CNSDocument1 pageMeghan Cule-Pathogens of The CNSMicroposterNo ratings yet
- The Urogenital Tract-Maija SwansonDocument1 pageThe Urogenital Tract-Maija Swansonmswanson5975No ratings yet
- Emily Scroggs Microorganisms Affecting The KidneyDocument1 pageEmily Scroggs Microorganisms Affecting The KidneyMicroposterNo ratings yet
- Wylie, ClareDocument1 pageWylie, ClareMicroposterNo ratings yet
- Liver PathogensDocument1 pageLiver PathogensMicroposterNo ratings yet
- Pathogens of The Gut - Christine ProchnowDocument1 pagePathogens of The Gut - Christine ProchnowMicroposterNo ratings yet
- Pathogens of The Lungs - Michelle CumbaaDocument1 pagePathogens of The Lungs - Michelle CumbaaMicroposterNo ratings yet
- Gaby Saenz - The MeningesDocument1 pageGaby Saenz - The MeningesMicroposterNo ratings yet
- Liver PathogensDocument1 pageLiver PathogensMicroposterNo ratings yet
- EarInfections FatimaKhalidDocument1 pageEarInfections FatimaKhalidMicroposterNo ratings yet
- Lung Infections - Jessica de AndaDocument1 pageLung Infections - Jessica de AndaMicroposterNo ratings yet
- Pathogens of The Female Reproductive System - Leah NechamkinDocument1 pagePathogens of The Female Reproductive System - Leah NechamkinMicroposterNo ratings yet
- Pathogens of The Vagina-Annie EspinosaDocument1 pagePathogens of The Vagina-Annie Espinosaannie_espinosa_2No ratings yet
- Pathogen (Poster Med Micro)Document1 pagePathogen (Poster Med Micro)joejackson6No ratings yet
- BlaC Genes and The Battle Against B-Lactamse in MDA TuberculosisDocument1 pageBlaC Genes and The Battle Against B-Lactamse in MDA TuberculosisMicroposterNo ratings yet
- Courtney Chinn - Skin InfectionsDocument1 pageCourtney Chinn - Skin InfectionsMicroposterNo ratings yet
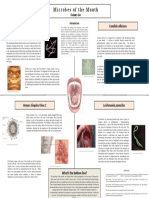
- Zach Lee - Mouth MicrobesDocument1 pageZach Lee - Mouth MicrobesMicroposterNo ratings yet
- Maladies of The Heart - Milan KantariaDocument1 pageMaladies of The Heart - Milan KantariaMicroposterNo ratings yet
- Actual NCPDocument10 pagesActual NCPRouie Björn ABrianNo ratings yet
- Ini Cet 22ND July 2021Document8 pagesIni Cet 22ND July 2021gksah711No ratings yet
- 180510-Jf-STARS Blackout Checklist FINALDocument6 pages180510-Jf-STARS Blackout Checklist FINALNithyaNo ratings yet
- Fundamentals of Nursing TestDocument78 pagesFundamentals of Nursing TestNicomille T. Caliging80% (5)
- Text Extraction Engine To Upgrade Clinical Decision Support SystemDocument4 pagesText Extraction Engine To Upgrade Clinical Decision Support SystemjournalNo ratings yet
- Borges, K. Et Al. (2018)Document7 pagesBorges, K. Et Al. (2018)Paulina Bermudez ValenzuelaNo ratings yet
- Dentistry and Community IUST 010-011Document72 pagesDentistry and Community IUST 010-011sharif100% (1)
- BJD 1348Document12 pagesBJD 1348Maciej BuczNo ratings yet
- Chest TubeDocument21 pagesChest TubeClaudio Nicolas Retamal YevenesNo ratings yet
- NIH Guidelines For Zoning of Hospitals 0701Document3 pagesNIH Guidelines For Zoning of Hospitals 0701SalehaQasimNo ratings yet
- Why Are Physicians and Laboratories Worried About BiotinDocument3 pagesWhy Are Physicians and Laboratories Worried About BiotinneofherNo ratings yet
- Metabolic Syndrome - Symptoms, Diagnosis, and CausesDocument7 pagesMetabolic Syndrome - Symptoms, Diagnosis, and CausesdrmuratNo ratings yet
- Health 6 Quarter 1 Module1Document12 pagesHealth 6 Quarter 1 Module1Cindy EsperanzateNo ratings yet
- Jurnal Alar FlareDocument10 pagesJurnal Alar FlareMayaSuyataNo ratings yet
- Mohamed Abdel Shafy Mohammady Tabl - 7 - Safety of Ticagrelor Post Fibrinolysis in STEMI PatientsDocument60 pagesMohamed Abdel Shafy Mohammady Tabl - 7 - Safety of Ticagrelor Post Fibrinolysis in STEMI PatientsJovita SardanisNo ratings yet
- Catholic University of America Student Health Plan For 2021-22 School YearDocument37 pagesCatholic University of America Student Health Plan For 2021-22 School YearThe College FixNo ratings yet
- Psychiatric Aspects of Diabetes MellitusDocument8 pagesPsychiatric Aspects of Diabetes MellitusKAUSHAL ARYANo ratings yet
- Chapter 23: RadiologyDocument23 pagesChapter 23: RadiologypoddataNo ratings yet
- EndocrineDocument49 pagesEndocrineApsaraNo ratings yet
- Slapped Cheek Syndrome (Part 2) - Hello DoktorDocument3 pagesSlapped Cheek Syndrome (Part 2) - Hello Doktornurhana humairaNo ratings yet
- Medication Dosing For Continuous Enteral Feedings Adult Inpatient 15.09.18 New LinksDocument30 pagesMedication Dosing For Continuous Enteral Feedings Adult Inpatient 15.09.18 New LinksMarsyaNo ratings yet
- Morphometric Study of Frontal Horn of Lateral Ventricles of The Brain by Computed Tomography in Western Up PopulationDocument6 pagesMorphometric Study of Frontal Horn of Lateral Ventricles of The Brain by Computed Tomography in Western Up Populationrhegi isdiara FairuzNo ratings yet
- The Gynaecological History Taking and Physical Examination: Associate Professor DR - Yin Moe HanDocument50 pagesThe Gynaecological History Taking and Physical Examination: Associate Professor DR - Yin Moe HanAimethevanNo ratings yet
- c4270f39-25e7-4dfb-873e-525de5f9ce16Document2 pagesc4270f39-25e7-4dfb-873e-525de5f9ce16ShivaS SNo ratings yet
- RomJOphthalmol 61 90Document5 pagesRomJOphthalmol 61 90Maria MiripNo ratings yet
- MCQ in General Surgery For UndergraduatesDocument259 pagesMCQ in General Surgery For UndergraduatesBadri Kobalava100% (5)
- Far Eastern University-Institute of Nursing In-House NursingDocument25 pagesFar Eastern University-Institute of Nursing In-House Nursingjonasdelacruz1111No ratings yet