Professional Documents
Culture Documents
Engineering Material-II: Iron Carbide Phase Diagram
Engineering Material-II: Iron Carbide Phase Diagram
Uploaded by
Ala Zi0 ratings0% found this document useful (0 votes)
13 views16 pagesThe document summarizes the iron-carbon phase diagram and the phases present in iron-carbon alloys. It describes:
1) The five main phases - ferrite, austenite, delta-ferrite, cementite, and liquid solution.
2) The four invariant reactions between the phases - peritectic, monotectic, eutectoid, and eutectic reactions.
3) How the microstructure of hypoeutectoid, eutectoid, and hypereutectoid steels depends on their carbon content when cooled slowly, forming either ferrite and cementite or pearlite.
Original Description:
material
Original Title
4_6014623350360378050
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document summarizes the iron-carbon phase diagram and the phases present in iron-carbon alloys. It describes:
1) The five main phases - ferrite, austenite, delta-ferrite, cementite, and liquid solution.
2) The four invariant reactions between the phases - peritectic, monotectic, eutectoid, and eutectic reactions.
3) How the microstructure of hypoeutectoid, eutectoid, and hypereutectoid steels depends on their carbon content when cooled slowly, forming either ferrite and cementite or pearlite.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
13 views16 pagesEngineering Material-II: Iron Carbide Phase Diagram
Engineering Material-II: Iron Carbide Phase Diagram
Uploaded by
Ala ZiThe document summarizes the iron-carbon phase diagram and the phases present in iron-carbon alloys. It describes:
1) The five main phases - ferrite, austenite, delta-ferrite, cementite, and liquid solution.
2) The four invariant reactions between the phases - peritectic, monotectic, eutectoid, and eutectic reactions.
3) How the microstructure of hypoeutectoid, eutectoid, and hypereutectoid steels depends on their carbon content when cooled slowly, forming either ferrite and cementite or pearlite.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 16
Engineering Material-II
Chapter One
Iron Carbide Phase Diagram
Compiled By: Abebe G.
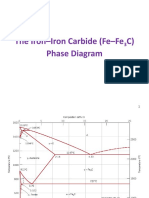
Iron Carbide Phase Diagram
• Iron-carbon phase diagram describes the iron-carbon system of
alloys containing up to 6.67% of carbon, discloses the phases
compositions and their transformations occurring with the alloys
during their cooling or heating.
• Carbon content 6.67% corresponds to the fixed composition of
the iron carbide Fe3C.
Fe-Fe C Phases
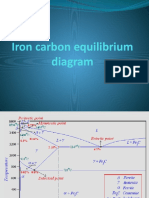
Phases in Fe–Fe3C Phase Diagram
-ferrite - solid solution of C in BCC Fe
• Stable form of iron at room temperature.
• The maximum solubility of C is 0.022 wt%
• Transforms to FCC -austenite at 912 C
-austenite - solid solution of C in FCC Fe
• The maximum solubility of C is 2.14 wt %.
• Transforms to BCC -ferrite at 1395 C
• Is not stable below the eutectoid temperature (727 C) unless
cooled rapidly.
Iron-iron Carbide phases
-ferrite solid solution of C in BCC Fe
• The same structure as -ferrite
• Stable only at high T, above 1394 C
• Melts at 1538 C
Fe3C (iron carbide or cementite)
• This intermetallic compound is metastable, it remains as
a compound indefinitely at room T, but decomposes
(very slowly, within several years) into -Fe and C
(graphite) at 650 - 700 C
Iron carbon phases
• Fe-Fe3C phase diagram is characterized by five individual
phases,:
• α–ferrite (BCC) Fe-C solid solution,
• γ-austenite (FCC) Fe-C solid solution,
• δ-ferrite (BCC) Fe-C solid solution,
• Fe3C (iron carbide) or cementite - an inter-metallic
compound and liquid Fe-C solution and
• four invariant reactions: peritectic, monotectic, eutectoid, &
eutectic reactions.
Iron iron-carbide phases
• peritectic reaction at 1495 C and 0.16%C, δ-ferrite + L↔ γ-iron
(austenite)
• Monotectic reaction 1495 C and 0.51%C, L ↔ L + γ-iron
(austenite)
• Eutectic reaction at 1147 C and 4.3 %C, L ↔ γ-iron +Fe3C
(cementite) [ledeburite]
• Eutectoid reaction at 723 C and 0.8%C, γ-iron ↔ α–ferrite
+Fe3C (cementite) [pearlite]
Phase compositions of the iron-carbon alloys at room
temperature
Hypoeutectoid steels (carbon content from 0 to 0.83%) consist of
primary (proeutectoid) ferrite (according to the curve A3) and pearlite.
Eutectoid steel (carbon content 0.83%) entirely consists of pearlite.
Hypereutectoid steels (carbon content from 0.83 to 2.06%) consist of
primary (proeutectoid) cementite (according to the curve ACM) and
pearlite.
Cast irons (carbon content from 2.06% to 4.3%) consist of
proeutectoid cementite C2 ejected from austenite according to the
curve ACM , pearlite and transformed ledeburite (ledeburite in which
austenite transformed to pearlite)
Mechanical properties:
Cementite is very hard and brittle - can strengthen steels.
Mechanical properties also depend on the microstructure,
that is, how ferrite and cementite are mixed.
Magnetic properties:
-ferrite is magnetic below 768 C, austenite is non-
magnetic
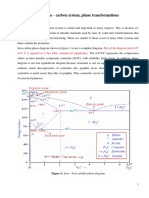
Development of microstructure in Fe C phases
•Microstructure depends on composition (carbon content) and heat
treatment. In the discussion below we consider slow cooling in which
equilibrium is maintained. = Microstructure of eutectoid steel (I)
Microstructure of eutectoid steel (II)
When alloy of eutectoid composition (0.76 wt % C) is cooled
slowly it forms perlite, a lamellar or layered structure of two
phases: -ferrite and cementite (Fe3C)
The layers of alternating phases in pearlite are formed for the same
reason as layered structure of eutectic structures: redistribution C
atoms between ferrite (0.022 wt%) and cementite (6.7 wt%) by
atomic diffusion.
Mechanically, pearlite has properties intermediate to soft, ductile
ferrite and hard, brittle cementite.
Microstructure of hypoeutectoid steel (III)
•Compositions to the left of eutectoid (0.022 - 0.76 wt % C)
hypoeutectoid (less than eutectoid –Greek word) alloys. +
+ Fe3C
The End of Chap 1
Thanks for your Attention
You might also like
- The Iron-Iron Carbide (Fe-Fe C) Phase DiagramDocument32 pagesThe Iron-Iron Carbide (Fe-Fe C) Phase DiagramNisaNo ratings yet
- Silicon ProductionDocument8 pagesSilicon ProductionMohamed EsmailNo ratings yet
- AASHTO M 270M 270-05 Structural Steel For BridgesDocument14 pagesAASHTO M 270M 270-05 Structural Steel For BridgesanjanaNo ratings yet
- Iron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingDocument7 pagesIron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingRajulapati Sunil KumarNo ratings yet
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongNo ratings yet
- 06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramDocument42 pages06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramTalaat Ahmed Mohamed El-Benawy100% (2)
- The IronCarbide DiagramDocument11 pagesThe IronCarbide DiagramshajjikhalidNo ratings yet
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- MEC 414 - Iron Phase Diagram Experiment 2Document7 pagesMEC 414 - Iron Phase Diagram Experiment 2boatcomNo ratings yet
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarNo ratings yet
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manNo ratings yet
- The Iron-Iron Carbide Equilibrium DiagramDocument15 pagesThe Iron-Iron Carbide Equilibrium DiagramjhangeerNo ratings yet
- Lesson 5 - Fe-C Diagram - Rev. 0Document11 pagesLesson 5 - Fe-C Diagram - Rev. 0Arga SetyaNo ratings yet
- 3 Iron Carbon DiaDocument21 pages3 Iron Carbon DiaChhavi SharmaNo ratings yet
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument58 pagesCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNo ratings yet
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 pagesCh-27.5 Iron Carbon Equilibrium DiagramManojNo ratings yet
- Iron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018Document30 pagesIron - Carbon Phase Diagram: Sandeep Nair CB - EN.P2MFG15018prasenjitsayantan100% (1)
- Iron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Document33 pagesIron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Mahmoud RefaatNo ratings yet
- Review MetalDocument38 pagesReview MetalnisannisaNo ratings yet
- Lec 7 Fe C DiagramDocument45 pagesLec 7 Fe C DiagramAdnan MehmoodNo ratings yet
- Phase Diagrams - 040823Document23 pagesPhase Diagrams - 040823Anthony MubangaNo ratings yet
- Iron Carbon Equilibrium DiagramDocument52 pagesIron Carbon Equilibrium DiagramSohan Lal100% (2)
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument79 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualdextrermachete4amgqgNo ratings yet
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument39 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (16)
- FeC and TTT DiagramsDocument12 pagesFeC and TTT DiagramsMohamed El-WakilNo ratings yet
- Fe C Phase DiagramDocument12 pagesFe C Phase DiagramDwi Cahyo NugrohoNo ratings yet
- Materials of Construction and Selection: Faculty of Chemical Engineering Universiti Teknologi MaraDocument80 pagesMaterials of Construction and Selection: Faculty of Chemical Engineering Universiti Teknologi MaraAisyah Addia AzizanNo ratings yet
- Unit-2 Notes (Material Science)Document24 pagesUnit-2 Notes (Material Science)AMAN SINGHNo ratings yet
- Ironiron CarbideequilibriumphasediagramDocument39 pagesIroniron CarbideequilibriumphasediagramSheikh UMARNo ratings yet
- EMM 2312 - Fe-CDocument53 pagesEMM 2312 - Fe-CCalebNo ratings yet
- Introduction-Iron Carbon Phase DiagramDocument31 pagesIntroduction-Iron Carbon Phase DiagramTHE BBEASTNo ratings yet
- Iron-Carbon Equilibrium DiagramDocument10 pagesIron-Carbon Equilibrium Diagrammissing wonder100% (1)
- Unit 6 (Phase &phase Transformations)Document14 pagesUnit 6 (Phase &phase Transformations)Beesam Ramesh KumarNo ratings yet
- Iron Carbon Note 1 2023Document23 pagesIron Carbon Note 1 2023gerrard samuelNo ratings yet
- Iron Iron-Carbide Equilibrium SystemDocument26 pagesIron Iron-Carbide Equilibrium SystemHiral HiraniNo ratings yet
- Unit Cell Cubic StructuresDocument8 pagesUnit Cell Cubic StructuresGuilherme Dos Santos MoreiraNo ratings yet
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Iron-Carbon Phase DiagramDocument30 pagesIron-Carbon Phase Diagramjunaid hassanNo ratings yet
- Phase Diagram of Fe-Fe3CDocument25 pagesPhase Diagram of Fe-Fe3CIram MustaviNo ratings yet
- MSM GTU Study Material E-Notes Unit-5 23112020052908AMDocument14 pagesMSM GTU Study Material E-Notes Unit-5 23112020052908AMVijayNo ratings yet
- IRON - CARBON DiagramDocument15 pagesIRON - CARBON Diagramgadde39100% (2)
- Engineering Materials 27-29Document40 pagesEngineering Materials 27-29Sanu SouravNo ratings yet
- Iron Carbon Diagram of Steel PDFDocument6 pagesIron Carbon Diagram of Steel PDFshihabscb1971100% (1)
- Iron Carbon Equilibrium DiagramDocument11 pagesIron Carbon Equilibrium Diagramganesh82No ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- EN1101 - MJE - Part 10 - Steel Phase Diagrams - LCDocument13 pagesEN1101 - MJE - Part 10 - Steel Phase Diagrams - LCnwankwo chubyNo ratings yet
- Plain Iron Carbon SteelsDocument5 pagesPlain Iron Carbon Steelsروشان فاطمة روشانNo ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- Iron Carbon Equillibrium Diagram GandhidhamDocument22 pagesIron Carbon Equillibrium Diagram Gandhidhamcal2_uniNo ratings yet
- Dokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dDocument16 pagesDokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dAfrizal Adithya PNo ratings yet
- Iron Carbon Diagram 6Document15 pagesIron Carbon Diagram 6Harris DarNo ratings yet
- Iron - Carbon SystemDocument21 pagesIron - Carbon SystemYavana KeerthiNo ratings yet
- Chapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMDocument13 pagesChapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMPAUL NDIRITUNo ratings yet
- PQT Chapter 9b Phase DiagramsDocument27 pagesPQT Chapter 9b Phase DiagramsDương Hữu PhươngNo ratings yet
- 03 - Iron - Iron CarbideDocument35 pages03 - Iron - Iron CarbidebotobotoakbarNo ratings yet
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 pagesSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserNo ratings yet
- The Iron-Carbon Phase DiagramDocument16 pagesThe Iron-Carbon Phase DiagramMeena SivasubramanianNo ratings yet
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Deep Earth: Physics and Chemistry of the Lower Mantle and CoreFrom EverandDeep Earth: Physics and Chemistry of the Lower Mantle and CoreHidenori TerasakiNo ratings yet
- The Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelRating: 5 out of 5 stars5/5 (4)
- Fundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionFrom EverandFundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionNo ratings yet
- Soldering (Acetate)Document11 pagesSoldering (Acetate)Maridee Bitalac AdiongNo ratings yet
- Referencias Bibliograficas PDFDocument3 pagesReferencias Bibliograficas PDFalchemist_fiqNo ratings yet
- Sheniblog-Sslc Chemistry-Chap 04 - Production of Metals - Previous Questions-EmDocument74 pagesSheniblog-Sslc Chemistry-Chap 04 - Production of Metals - Previous Questions-Embattlegroundmobileindia815No ratings yet
- Treatment of A Liquid AluminumDocument55 pagesTreatment of A Liquid AluminumLilian Jefferson Malavazi100% (1)
- FP - EN - Polybio 630 CU - BB - 1114 - 0Document1 pageFP - EN - Polybio 630 CU - BB - 1114 - 0tribolasNo ratings yet
- Engineering Review MaterialsDocument7 pagesEngineering Review MaterialsJerick HernandezNo ratings yet
- Additive Manufacturing: H.R. Kotadia, Associate Professor, G. Gibbons, A. Das, P.D. HowesDocument23 pagesAdditive Manufacturing: H.R. Kotadia, Associate Professor, G. Gibbons, A. Das, P.D. HowesÁlvaro Nieto CastroNo ratings yet
- 6005a t6 Extrusion TCDocument4 pages6005a t6 Extrusion TCKhamda Aja DuluNo ratings yet
- Inoculation of Ductile Iron Why and WhenDocument4 pagesInoculation of Ductile Iron Why and WhenKarthiKeyan SNo ratings yet
- Aircraft Materials and ProcessesDocument15 pagesAircraft Materials and ProcessesRajesh KumarNo ratings yet
- JP002 Tin Whiskers TheoryDocument30 pagesJP002 Tin Whiskers Theorydemolinux100% (3)
- Ductile IronDocument2 pagesDuctile Ironpraval84No ratings yet
- Homework 10Document2 pagesHomework 10FrknNo ratings yet
- Consumables: Welding Inspection Welding InspectionDocument5 pagesConsumables: Welding Inspection Welding Inspectionpaeg6512No ratings yet
- Vci Zipper BagsDocument2 pagesVci Zipper BagsBugNo ratings yet
- 13CRMO44Document2 pages13CRMO44stamatsNo ratings yet
- Materials List: Alloy FamilyDocument3 pagesMaterials List: Alloy FamilydiwakarNo ratings yet
- Fake Ash Laura's Turquoise Based TestsDocument8 pagesFake Ash Laura's Turquoise Based TestsTim CarlsonNo ratings yet
- 01-Metals & Non MetalsDocument23 pages01-Metals & Non Metalssandeep kumar yadavNo ratings yet
- FAQ Processes For Surface Hardening of Stainless Steels Bodycote S3PDocument4 pagesFAQ Processes For Surface Hardening of Stainless Steels Bodycote S3PSinan YıldızNo ratings yet
- Duplex Stainless Steel Quality - ASTM A923 Vs ISO 17781: HistoryDocument4 pagesDuplex Stainless Steel Quality - ASTM A923 Vs ISO 17781: HistoryshojiNo ratings yet
- CREEP Corrosion Presentation MS2007Document4 pagesCREEP Corrosion Presentation MS2007anwarhas05No ratings yet
- Heat Treatment of SteelsDocument162 pagesHeat Treatment of SteelsINSTECH ConsultingNo ratings yet
- HQ182EN Metal FormingDocument6 pagesHQ182EN Metal FormingLuis MartinezNo ratings yet
- ALUMERO - Alloys EN AW 6060 AlMgSi0 - WebDocument1 pageALUMERO - Alloys EN AW 6060 AlMgSi0 - WebemilasanovskiNo ratings yet
- Property and Microstructure Evaluation As A Function of Processing Parameters: Large HY-80 Steel Casting For A US Navy SubmarineDocument13 pagesProperty and Microstructure Evaluation As A Function of Processing Parameters: Large HY-80 Steel Casting For A US Navy SubmarineKay WhiteNo ratings yet
- Lion 26: PT. Lion Superior ElectrodesDocument1 pageLion 26: PT. Lion Superior ElectrodesJulius HendraNo ratings yet
- Metals and How To Weld Them-The James F. Lincoln Arc Welding FoundationDocument392 pagesMetals and How To Weld Them-The James F. Lincoln Arc Welding FoundationHammad Ashraf100% (1)