Professional Documents
Culture Documents
Poster V1
Poster V1
Uploaded by
Trang ĐoànOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Poster V1
Poster V1
Uploaded by
Trang ĐoànCopyright:
Available Formats
Tên trường
Ngày tháng năm
Investigation of Oyster Shell Powder and Zero-valent Iron as
bioinfiltration media to remove heavy metals from Stormwater
Trung Nguyen Thanh1, Po-Hsun Lin2
1
Department of Safety, Health and Environmental Engineering, Ming Chi University of Technology
2
Department of Materials Engineering, Ming-Chi University of Technology
*Corresponding author. E-mail address: phlin@mail.mcut.edu.tw
Abstract
In Taiwan, the huge amounts of abandoned oyster shells have caused problems including their noxious odor and illegal dumping into the sea. It is an urgent requirement,
therefore, to find environmentally safe and profitable uses for waste oyster shell. This paper presents a column study conducted to investigate the potential use of Oyster
Shell Powder (OSP) and Zero-Valent Iron (ZVI) and manganese oxide coated oyster-shell powder (MOCOSP) as filter medium for treatment of heavy metal in stormwater.
Three PVC laboratory-scale columns (4 cm diameter, 40 cm deep) were carried out under dynamic flow conditions and packed with 25cm depth MOCOSP (0.84 – 1 mm),
the mixture of sieved ZVI (1 mm) and sieved OSP (0.84 – 1 mm) in the ratio of 1 to 10 (by weight) and sieved OSP (0.84 – 1 mm) and, respectively. Synthetic stormwater
including Cu, Ni, Pb, Zn was used to introduce to the columns at a flow velocity at 1.67 cm/min. All three columns demonstrated good ability to remove heavy metals
followed the order of (MOCOSP)>(OSP+ZVI)>(OSP), especially Cu and Pb , followed by Zn and Ni. In addition, the removal efficiencies were in the steady decline over
time. The results suggested that OSP is no longer waste, so we fully utilize it as an absorbent for treating heavy metals in water.
Material and methods Results
1. Oyster-shell powder The detailed removal efficiency of Cu(II), Ni(II), Pb(II) and Zn(II) of each
Oyster-shell wastes were washed and at 105oC for 3 hours and dried shells were media (column) are shown on Figure 4, 5, 6, and 7, respectively.
then crushed by hammer and crusher prior to sieving through two sieves of
1.190 and 0.840 mm (figure 1c). 100 100
90
2. Manganese oxide coated oyster-shell powder (MOCOSP)
Removal of Ni by MOCOSP
90
80 Removal of Ni by OSP+ZVI
Removal of Ni by OSP
Removal efficiency (%)
Removal efficiency (%)
70
OSP was base activated to improve the specific surface area. Washed OSP was 80
60
suspended in NaOH solution for 24 hours, then washed with DI water and dried 70 50
in an oven at 105 oC for 5 hours. The prepared base-activated OSP was 60
40
suspended in KMnO4 solution for 24 hours, then calcinated in a muffle furnace
30
Removal of Cu by MOCOSP 20
50
at 250 oC for 4 hours. The KMnO4 was decomposed (Reaction Equation (1)). Removal of Cu by OSP+ZVI
Removal of Cu by OSP 10
40 0
2KMnO 4 K 2 MnO 4 MnO 2 O 2 (Equation 1) 0 20 40 60 80 100 120 140 160 180 200 0 20 40 60 80 100 120 140 160 180 200
Time (hours) Time (hours)
Figure 4: Removal efficiency of Cu Figure 5: Removal efficiency of Ni
OSP
DI water
100 100
Washed OSP 90
Dried 105 C 5 hours
80
o 90
Figure 1a: Washed OSP
Removal efficiency (%)
Removal efficiency (%)
70
Dried OSP 80
60
50
NaOH (9%) 24 hours 40
30
70
105 oC 5 hours Removal of Pb by MOCOSP
Removal of Pb by OSP+ZVI
20
Removal of Zn by MOCOSP
Removal of Zn by OSP+ZVI
Removal of Pb by OSP 10
Removal of Zn by OSP
Figure 1b: Crushed OSP
60 0
KMnO4 (7%) 24 hours 0 20 40 60 80 100 120 140 160 180 200 0 20 40 60 80 100 120 140 160 180 200
Time (hours) Time (hours)
Figure 6: Removal efficiency of Pb Figure 7: Removal efficiency of Zn
Calcination 250 oC 4 hours
MOCOSP
Figure 1c: OSP Figure 2: Process of making MOCOSP
Conclusions
In this study, the adsorption potential of OSP was investigated for the removal
3. Column experiments of Cu(II), Ni(II), Pb(II) and Zn(II) from aqueous systems. The kinetic of Cu(II),
Ni(II), Pb(II) and Zn(II) onto media are study with three columns. The
Three PVC columns (4 cm diameter, 40 cm deep) were carried out and packed following results are obtained:
with 25 cm depth MOCOSP, the mixture of sieved ZVI (1 mm) and OSP in the
1. All three columns show the ability to remove heavy metals well but
ratio of 1 to 10 (by weight) and OSP, respectively. Flow velocity was 1.67
tend to decrease over time.
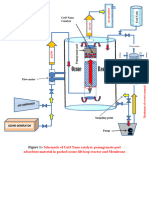
cm/min. Experimental diagram is designed as figure 3.
2. MOCOSP and the mixture of OSP and ZVI exhibited significantly
The heavy metal solution was prepared using the following salts: PbCl2, ZnCl2,
better heavy metal removal efficiency then OSP, especially Pb(II) and
NiCl2, Cu(NO3)2 with few drops concentrated HNO3 in order to prevent the
Cu(II).
precipitation by hydrolysis. The concentration of Pb(II), Zn(II), Ni(II) and
Cu(II) are 1 ppm, 2 ppm, 1 ppm and 1 ppm, respectively. 3. MOCOSP demonstrated the most outstanding and most durable metal
removal capacity. It is worth noting that MOCOSP is a potential media
used to removal Nickel from water.
4. OSP is no longer waste, so we fully utilize it as an absorbent for
treating heavy metals in water.
Acknowledgements
The authors express their sincere gratitude to Ming Chi University of Technology
(MCUT) in Taiwan for the financial support and Wen-Hui Kuan’s lab (MCUT) for
Figure 3: Column experiment helping with the heavy metal analysis.
www.postersession.com
www.postersession.com
You might also like
- Case1 - TelenorDocument4 pagesCase1 - TelenorMine Sayrac67% (3)
- Lab Proj.05 Hex EditorDocument13 pagesLab Proj.05 Hex EditorQuốc Việt NguyễnNo ratings yet
- RSC Advances: PaperDocument9 pagesRSC Advances: Paperdevi eraNo ratings yet
- Portale Et Al., 2023Document5 pagesPortale Et Al., 2023ventilabroNo ratings yet
- One-Step Preparation and Characterization of Zinc Phosphate Nanocrystals With Modified SurfaceDocument5 pagesOne-Step Preparation and Characterization of Zinc Phosphate Nanocrystals With Modified SurfaceDương Minh MẫnNo ratings yet
- Blasting Optimization in A Phosphate Rock Mine in BrazilDocument10 pagesBlasting Optimization in A Phosphate Rock Mine in BrazilAndré Carlos SilvaNo ratings yet
- The SuCy Process SulfatosDocument18 pagesThe SuCy Process SulfatoslauramanjarresNo ratings yet
- Vélez 2016 J. Phys.%3A Conf. Ser. 687 012050Document5 pagesVélez 2016 J. Phys.%3A Conf. Ser. 687 012050Ysabelle JimeneaNo ratings yet
- Fadillah 2021 J. Phys. - Conf. Ser. 1816 012019Document8 pagesFadillah 2021 J. Phys. - Conf. Ser. 1816 012019Ari Sulistyo RIniNo ratings yet
- Lab P06 - Boron Ion Implantation and AnnealingDocument1 pageLab P06 - Boron Ion Implantation and AnnealingAnh LuuNo ratings yet
- Studies On Regenerated Protein Fibers. Production Regenerated Fibroin Fiber by The Self-Dialyzing Wet Spinning MethodDocument9 pagesStudies On Regenerated Protein Fibers. Production Regenerated Fibroin Fiber by The Self-Dialyzing Wet Spinning Methodapi-3733260No ratings yet
- Yang Hu, John F. Honek, and Qing-Bin Lu Department of Physics and Astronomy, and Department of Chemistry, University of WaterlooDocument1 pageYang Hu, John F. Honek, and Qing-Bin Lu Department of Physics and Astronomy, and Department of Chemistry, University of WaterlooYang HuNo ratings yet
- DR Sulbha Kulkarni-1Document48 pagesDR Sulbha Kulkarni-1api-20005351100% (1)
- Hydrometallurgy: Yuna Zhao, Guocai Zhu, Zhuo ChengDocument7 pagesHydrometallurgy: Yuna Zhao, Guocai Zhu, Zhuo ChengYuliantari YuliantariNo ratings yet
- Solvothermal Derived Crystalline NiOx Nanoparticles For High Performance Perovskite Solar CellsDocument8 pagesSolvothermal Derived Crystalline NiOx Nanoparticles For High Performance Perovskite Solar CellsPam S. PowellNo ratings yet
- Removal Properties of Low-Thermal-Expansion Materials Withrotating-Sphere Elastic Emission MachiningDocument4 pagesRemoval Properties of Low-Thermal-Expansion Materials Withrotating-Sphere Elastic Emission MachiningLucas HarimNo ratings yet
- 20HDP Template SuCyProcess v0Document18 pages20HDP Template SuCyProcess v0Mario Cancino SerranoNo ratings yet
- Geopedologi2021 - LDS - OreDeposits3 - Nickel LateriteDocument19 pagesGeopedologi2021 - LDS - OreDeposits3 - Nickel LateriteRidho FirdausmanNo ratings yet
- 1 Ammonia Aop TiO2Document6 pages1 Ammonia Aop TiO2achmadinNo ratings yet
- Unit 4Document50 pagesUnit 4siva prasadNo ratings yet
- CMP2015 - Nanoparticle Flotation Aids For Pentlandite FinesDocument13 pagesCMP2015 - Nanoparticle Flotation Aids For Pentlandite FinesrodrigoNo ratings yet
- Appnote Targeted Removal of Metallic Contamination 8586 PDFDocument4 pagesAppnote Targeted Removal of Metallic Contamination 8586 PDFImah Shetye New'zethaNo ratings yet
- Hydrometallurgy: Sait Kursunoglu, Zela Tanlega Ichlas, Muammer KayaDocument7 pagesHydrometallurgy: Sait Kursunoglu, Zela Tanlega Ichlas, Muammer KayaDiego GutiérrezNo ratings yet
- Science & Technology Indonesia: CALCIUM OXIDE FROM Pomacea Canaliculata AND Babylonia Spirata SNAILSDocument3 pagesScience & Technology Indonesia: CALCIUM OXIDE FROM Pomacea Canaliculata AND Babylonia Spirata SNAILSrisfiNo ratings yet
- ME189 - Chapter 8 PDFDocument56 pagesME189 - Chapter 8 PDFgauravkumar bhandari100% (1)
- Art 7 OriginalDocument6 pagesArt 7 OriginalDiego PalominoNo ratings yet
- Seyer 2001Document4 pagesSeyer 2001malcolmclark224No ratings yet
- Sian Ournal of HemistryDocument3 pagesSian Ournal of HemistryMara Bangun HarahapNo ratings yet
- Working Principle Powder CharacteristicsDocument2 pagesWorking Principle Powder CharacteristicsMuthu KumarNo ratings yet
- Sun Qing 2000Document8 pagesSun Qing 2000Thiago OliveiraNo ratings yet
- Ultraviolet-Visible Spectral Properties of Nanometer Zinc Oxide Colloidal SolutionDocument4 pagesUltraviolet-Visible Spectral Properties of Nanometer Zinc Oxide Colloidal SolutionAnonymous cYpEVvoNo ratings yet
- Literature SurveyDocument10 pagesLiterature SurveyNeil DiasNo ratings yet
- Tunablemorphologies OzcanDocument7 pagesTunablemorphologies OzcanjoseNo ratings yet
- Fyp Poster r5Document1 pageFyp Poster r5Muhammad Zaib KhanNo ratings yet
- A Monolayer Dispersion Study of Titania-Supported Copper OxideDocument6 pagesA Monolayer Dispersion Study of Titania-Supported Copper OxideGuiexhoba MedranoNo ratings yet
- Bainite Obtaining CIDocument6 pagesBainite Obtaining CIashokjkhannaNo ratings yet
- 1 s2.0 0016236183903046 MainDocument6 pages1 s2.0 0016236183903046 Mainrenata dias e silvaNo ratings yet
- Extraction of Niobium and TantalumDocument6 pagesExtraction of Niobium and TantalumTamia ObaidNo ratings yet
- 1 s2.0 S0921344920307060 MainDocument8 pages1 s2.0 S0921344920307060 MainmarfelmagNo ratings yet
- Nitrogen Family Chemistry NotesDocument65 pagesNitrogen Family Chemistry NotesMoningi SrijaNo ratings yet
- Recovery of Nickel and Cobalt From Laterite Leach Tailings Through Resin-In-Pulp Scavenging and Selective Ammoniacal Elution PDFDocument7 pagesRecovery of Nickel and Cobalt From Laterite Leach Tailings Through Resin-In-Pulp Scavenging and Selective Ammoniacal Elution PDFRodrigoNo ratings yet
- Performance Evaluation of Activated Neem Bark for the Removal of Zn(II) and Cu(II) Along With Other Metal Ions From Aqueous Solution and Synthetic Pulp & Paper Industry Effluent Using Fixed-bed ReactorDocument11 pagesPerformance Evaluation of Activated Neem Bark for the Removal of Zn(II) and Cu(II) Along With Other Metal Ions From Aqueous Solution and Synthetic Pulp & Paper Industry Effluent Using Fixed-bed ReactorUtkarsh MaheshwariNo ratings yet
- Biological Nutrient Removal in A Small-Scale MBR Treating PDFDocument9 pagesBiological Nutrient Removal in A Small-Scale MBR Treating PDFAlejandra Medina ArmijoNo ratings yet
- This Content Downloaded From 150.161.96.12 On Mon, 19 Dec 2022 17:22:57 UTCDocument6 pagesThis Content Downloaded From 150.161.96.12 On Mon, 19 Dec 2022 17:22:57 UTCREGINA BUARQUE DE GUSMAONo ratings yet
- Ref - Ozone - KGK - 2018 - 03 - 32-36Document6 pagesRef - Ozone - KGK - 2018 - 03 - 32-36Chanin NgudsuntearNo ratings yet
- Bulk FlotationDocument6 pagesBulk FlotationisepcontrolNo ratings yet
- 2016 Synthesis of TiO2 and ZnO Nano and Submicrometric Fibers by Solution Blow SpinningDocument5 pages2016 Synthesis of TiO2 and ZnO Nano and Submicrometric Fibers by Solution Blow SpinningEliton Medeiros Candido de MacêdoNo ratings yet
- 18 - Shen2008, Photocatalytic Degradation For Methylene Blue Using Zinc Oxide PreparedDocument4 pages18 - Shen2008, Photocatalytic Degradation For Methylene Blue Using Zinc Oxide Preparedhellna284No ratings yet
- Physics MCQs Booklet X PDFDocument24 pagesPhysics MCQs Booklet X PDFMarvel StudioNo ratings yet
- 4.02 IR SpectrosDocument23 pages4.02 IR SpectrosJoeNo ratings yet
- Vishwanath InternshipDocument15 pagesVishwanath Internshipvishwanathhugar25No ratings yet
- Shantel ProjectDocument5 pagesShantel ProjectAgelaga VictorNo ratings yet
- Doared Uor, Dnod, D:Od9: Karnataka Pollution ControlDocument7 pagesDoared Uor, Dnod, D:Od9: Karnataka Pollution ControlAnonymous oUoJ4A8xNo ratings yet
- 12 Nanofiltration and Reverse OsmosisDocument57 pages12 Nanofiltration and Reverse OsmosisBill644No ratings yet
- PVT Systems-Sustainability - SSSDocument67 pagesPVT Systems-Sustainability - SSSSnehal AbhyankarNo ratings yet
- E. Poster 23017002 IntanDocument1 pageE. Poster 23017002 IntannatayaNo ratings yet
- منظومة البحث الثاني PDFDocument1 pageمنظومة البحث الثاني PDFali abdulrahman al-ezziNo ratings yet
- Beneficiation of Kankara KaolinDocument9 pagesBeneficiation of Kankara KaolinLAWRENCE OTUNo ratings yet
- Lithography: Overview of Process StepsDocument46 pagesLithography: Overview of Process StepsPrakharNo ratings yet
- Selective Extraction of Cobalt From Nickel Sulphate Solutions by CyanexDocument6 pagesSelective Extraction of Cobalt From Nickel Sulphate Solutions by CyanexArifo Gunawan CahyanegoroNo ratings yet
- Smart Light-Responsive Materials: Azobenzene-Containing Polymers and Liquid CrystalsFrom EverandSmart Light-Responsive Materials: Azobenzene-Containing Polymers and Liquid CrystalsNo ratings yet
- Oxford English Lifetime Level 2 Student - S BookDocument66 pagesOxford English Lifetime Level 2 Student - S Bookthanh vo huynh100% (1)
- Free Download Autodesk AutoCAD Mechanical 2018.1 Full CrackDocument16 pagesFree Download Autodesk AutoCAD Mechanical 2018.1 Full CrackAzmi Bin A Matali100% (1)
- 9781803243450-Template Metaprogramming With CDocument480 pages9781803243450-Template Metaprogramming With CpixelfruitsstudioNo ratings yet
- Bangalore District - Delers - ManufacturersDocument14 pagesBangalore District - Delers - ManufacturersGautham SukNo ratings yet
- Uni Stuttgart DissertationenDocument5 pagesUni Stuttgart DissertationenOrderCustomPapersSingapore100% (1)
- Fnexam 1Document11 pagesFnexam 1kaye castilloNo ratings yet
- TESDA Circular No. 051-2021Document56 pagesTESDA Circular No. 051-2021jenneath chuaNo ratings yet
- Com Power Lvmkt215enDocument70 pagesCom Power Lvmkt215enCbdtxd PcbtrNo ratings yet
- Paroc-Fire-Protection-Guide1-Steel-UK 3005-File076172 PDFDocument10 pagesParoc-Fire-Protection-Guide1-Steel-UK 3005-File076172 PDFNenad GajicNo ratings yet
- Micro PythonDocument21 pagesMicro PythonGiani BuzatuNo ratings yet
- Notes 27 6 2023Document2 pagesNotes 27 6 2023Saksham Bansal X-CNo ratings yet
- D. (4) Answer (1) and (2) Are CorrectDocument22 pagesD. (4) Answer (1) and (2) Are CorrectJAWAD100% (1)
- HASEE HP500 - QUANTA SW7 - REV 1ASecDocument41 pagesHASEE HP500 - QUANTA SW7 - REV 1ASecAdam TsiolakakisNo ratings yet
- TDS Meyco Sa 167 PDFDocument3 pagesTDS Meyco Sa 167 PDFYasin AykanatNo ratings yet
- Song 2017Document5 pagesSong 2017201400738No ratings yet
- Mathematics 9Document5 pagesMathematics 9Van Mark TejadaNo ratings yet
- Ligaduras Griegas - WikipediaDocument10 pagesLigaduras Griegas - WikipediaCarlos Arturo MedinaNo ratings yet
- The Invigilator App - Student User Guide - UNISADocument20 pagesThe Invigilator App - Student User Guide - UNISAButhelezi ThobekaNo ratings yet
- 6513de (T8001ub Diagrama TV EmersonDocument69 pages6513de (T8001ub Diagrama TV Emersonwilson de jesus miranda noreñaNo ratings yet
- Lecture 3 CADDocument10 pagesLecture 3 CADdesubie bireNo ratings yet
- IIT M DIPLOMA ET1 EXAM QPD1 S2 30 Apr 2023Document380 pagesIIT M DIPLOMA ET1 EXAM QPD1 S2 30 Apr 2023Riya PandeyNo ratings yet
- Autumn Break Holidays Homework 2021-22Document3 pagesAutumn Break Holidays Homework 2021-22siddharthkumar pandeyNo ratings yet
- Challenge of Artificial IntelligenceDocument230 pagesChallenge of Artificial IntelligenceDragosNo ratings yet
- Service Manual: DJ-596T / DJ-596EDocument37 pagesService Manual: DJ-596T / DJ-596ERidwan SsrNo ratings yet
- Unit 9 RevisionDocument5 pagesUnit 9 Revisionl wanningNo ratings yet
- ICH E6-R3 GCP-Principles Draft 2021 0419Document7 pagesICH E6-R3 GCP-Principles Draft 2021 0419ramya sandraNo ratings yet
- Workmode API in FRUNDocument16 pagesWorkmode API in FRUNGuillaume LavoixNo ratings yet