Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
9 viewsUntitled
Untitled
Uploaded by
RAVI5268Lower cycloalkanes like cyclopropane and cyclobutane experience more angle strain due to deviations from the normal tetrahedral bond angle of 109.5°. This makes them more reactive and prone to ring-opening reactions like halogenation, hydrogenation, and reactions with hydrogen halides. Baeyer's strain theory proposed that angle strain increases with greater deviations from the optimal 109.5° bond angle and influences the relative stability and reactivity of cycloalkanes. Later theories such as bent bonds and puckered ring conformations provided alternative explanations for the reactivity of small ring cycloalkanes.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You might also like
- Unit 5Document42 pagesUnit 5RAVI5268No ratings yet
- Cycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheoryDocument5 pagesCycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheorySayed AltamashNo ratings yet
- Guru Nanak Institute of Pharmaceutical Science and TechnologyDocument13 pagesGuru Nanak Institute of Pharmaceutical Science and TechnologySomali SenguptaNo ratings yet
- Pharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GDocument11 pagesPharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GSujanNo ratings yet
- Baeyrs Strain TheoryDocument5 pagesBaeyrs Strain TheorySk ZNo ratings yet
- Relative Stabilities of CycloakanesDocument8 pagesRelative Stabilities of CycloakanesKamal KishoreNo ratings yet
- What Are Cyclo-Alkanes?Document6 pagesWhat Are Cyclo-Alkanes?gfgfccNo ratings yet
- Cyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Document6 pagesCyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Tushar GiradkarNo ratings yet
- Cyclic Aliphatic Compounds: NomenclatureDocument19 pagesCyclic Aliphatic Compounds: NomenclatureWinnie SantiagoNo ratings yet
- Tutorial07b OrganicCycloalkanesDocument24 pagesTutorial07b OrganicCycloalkanesHana NisrinaNo ratings yet
- Chemistry PDFDocument9 pagesChemistry PDFRájésh KùmârNo ratings yet
- Cyclo AlkanesDocument38 pagesCyclo Alkanesdinesh111180No ratings yet
- Cyclo Al KanesDocument3 pagesCyclo Al KanesIbraim ZekirNo ratings yet
- LecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryDocument8 pagesLecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryKevalNo ratings yet
- Coulson and MoffittDocument2 pagesCoulson and MoffittTanya 60No ratings yet
- Lesson StructureDocument19 pagesLesson Structurer karthickNo ratings yet
- 3.4 The Shapes of Cycloalkanes: Planar or Nonplanar?Document29 pages3.4 The Shapes of Cycloalkanes: Planar or Nonplanar?juanNo ratings yet
- Cyclic Alkanes & AlkenesDocument18 pagesCyclic Alkanes & AlkenesbgbscgvNo ratings yet
- Conformational Analysis of CyclopentaneDocument11 pagesConformational Analysis of CyclopentaneNimra MalikNo ratings yet
- Conformational Analysis For StudentsDocument89 pagesConformational Analysis For Studentsindrapal kumarNo ratings yet
- SCH 102 Lecture Week 8 2018-9Document27 pagesSCH 102 Lecture Week 8 2018-9Radhika TopwalNo ratings yet
- Chapter 4Document33 pagesChapter 4채종희No ratings yet
- MODULE 5: Conformational Analysis of Alkanes and CyclohexanesDocument8 pagesMODULE 5: Conformational Analysis of Alkanes and CyclohexanesARMAN AKRAM BIN OMAR / UPMNo ratings yet
- Conformational AnalysisDocument10 pagesConformational AnalysisPG Chemistry PG ChemistryNo ratings yet
- Conformation AnalysisDocument41 pagesConformation AnalysisJoseNo ratings yet
- Angle Strain Torsional Strain Ring StrainDocument6 pagesAngle Strain Torsional Strain Ring StrainchuasioklengNo ratings yet
- InorganicDocument43 pagesInorganicShobhit ZakhmiNo ratings yet
- Chapter 4. Alkanes (BSChem)Document59 pagesChapter 4. Alkanes (BSChem)Chase PattersonNo ratings yet
- Chapter 4 With Video LinksDocument37 pagesChapter 4 With Video LinksDoom RefugeNo ratings yet
- Organic Chemistry: M. R. Naimi-JamalDocument81 pagesOrganic Chemistry: M. R. Naimi-JamalHamidatun NisaNo ratings yet
- Reaction MechanismDocument30 pagesReaction Mechanismantoniostark0010No ratings yet
- 1515564149CHE P1 M16 EtextDocument22 pages1515564149CHE P1 M16 EtextElangovan NatarajanNo ratings yet
- GeneralChem LS 25 PDFDocument25 pagesGeneralChem LS 25 PDFSunil NahataNo ratings yet
- UntitledDocument46 pagesUntitled양우경No ratings yet
- Lecture 31 PDFDocument17 pagesLecture 31 PDFRachit ShahNo ratings yet
- DecalinsDocument25 pagesDecalinstessyNo ratings yet
- Conformation & Conformational IsomersDocument4 pagesConformation & Conformational Isomerspulkit asatiNo ratings yet
- CHEM 210-1 Organic Chemistry NotesDocument26 pagesCHEM 210-1 Organic Chemistry NotesRobert GardnerNo ratings yet
- Alkanes: Sakti Hidayati FikriyaDocument26 pagesAlkanes: Sakti Hidayati FikriyaShinta Novita SariNo ratings yet
- Cyclohexane Conformational AnalysisDocument1 pageCyclohexane Conformational Analysis휘승No ratings yet
- Stereo KIMIA, KIMIA ORGANIK 2Document92 pagesStereo KIMIA, KIMIA ORGANIK 2Mutia HadiyantiNo ratings yet
- Chapter 3Document27 pagesChapter 3Siddarth PalletiNo ratings yet
- Alkanes, Alkenes, Alkynes, and CycloalkanesDocument162 pagesAlkanes, Alkenes, Alkynes, and CycloalkanesNurtasha AtikahNo ratings yet
- Carbocations 2Document16 pagesCarbocations 2Surabhi BansalNo ratings yet
- Monocyclic DisubstitutedDocument3 pagesMonocyclic DisubstitutedPs SatchithNo ratings yet
- محاضرات العضوية-1-الثالثةDocument13 pagesمحاضرات العضوية-1-الثالثةlindanenkosiNo ratings yet
- Chapter 3: Structure and Stereochemistry of AlkanesDocument42 pagesChapter 3: Structure and Stereochemistry of AlkanesKera Maria AllengerNo ratings yet
- StudentDocument31 pagesStudentnanaNo ratings yet
- Organic - Class 8Document41 pagesOrganic - Class 8Sajan Singh LUCKYNo ratings yet
- Cyclic AlkanesDocument35 pagesCyclic AlkanesKunjal100% (2)
- Conformaciones Diferentes Configuraciones DiferentesDocument45 pagesConformaciones Diferentes Configuraciones DiferentesJuan CarlosNo ratings yet
- Eme104 Lecture 2Document8 pagesEme104 Lecture 2grantwekesaNo ratings yet
- Conformational IsomersDocument8 pagesConformational Isomersshirodkar_16593No ratings yet
- BSES Topic 06 Cycloalkanes and Aromatic CompoundsDocument42 pagesBSES Topic 06 Cycloalkanes and Aromatic CompoundsJhunessa Mae JalagatNo ratings yet
- Organic Chemistry TermsDocument7 pagesOrganic Chemistry TermssbelodoNo ratings yet
- Conformational Analysis:: Chapter - 6 Stereochemistry NotesDocument12 pagesConformational Analysis:: Chapter - 6 Stereochemistry NotesMohit KambojNo ratings yet
- Organic Chemistry TutorialsDocument11 pagesOrganic Chemistry TutorialsSuyash OnkarNo ratings yet
- Conformations IIDocument16 pagesConformations IIsndNo ratings yet
- U-4, Poc-I, Carewell PharmaDocument19 pagesU-4, Poc-I, Carewell PharmaRAVI5268No ratings yet
- Unit 5Document42 pagesUnit 5RAVI5268No ratings yet
- UJPBS 23305 (RS)Document6 pagesUJPBS 23305 (RS)RAVI5268No ratings yet
- POC I Practical Carewell PharmaDocument27 pagesPOC I Practical Carewell PharmaRAVI5268No ratings yet
- ChatLog Online Workshop On Child Rights and Protection For Coordinators - Officers of National Service Scheme - NSS - Date - 19 - 20 August2020 - Timings 3 - 00PM - 5 - 00PM - 2020-08-19 16 - 19Document2 pagesChatLog Online Workshop On Child Rights and Protection For Coordinators - Officers of National Service Scheme - NSS - Date - 19 - 20 August2020 - Timings 3 - 00PM - 5 - 00PM - 2020-08-19 16 - 19RAVI5268No ratings yet
- E HealthDocument57 pagesE HealthRAVI5268100% (2)
- DTMM and COSMIC Molecular Mechanics Parameters For AlkylsilanesDocument14 pagesDTMM and COSMIC Molecular Mechanics Parameters For AlkylsilanesLuu Xuan CuongNo ratings yet
- Neral Organic Chemistry (62-80)Document19 pagesNeral Organic Chemistry (62-80)udaysrinivasNo ratings yet
- Important Concepts: Conformational Analysis of AlkanesDocument4 pagesImportant Concepts: Conformational Analysis of AlkanesKayla JoezetteNo ratings yet
- Conformational AnalysisDocument53 pagesConformational AnalysisDavidAlejoNo ratings yet
- CH 4 AlkanesDocument16 pagesCH 4 AlkanesBrittnay Marie100% (4)
- Stereochemistry of Organic CompoundsDocument31 pagesStereochemistry of Organic CompoundsSrinivasulu KonetiNo ratings yet
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Document8 pagesChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNo ratings yet
- Chem 210 PSU Exam 1Document7 pagesChem 210 PSU Exam 1milkah mwauraNo ratings yet
- StereochemistryDocument108 pagesStereochemistryAllan DNo ratings yet
- Fiitjee Test PaperDocument23 pagesFiitjee Test PaperNikita GargNo ratings yet
- DimethylpananthereneDocument11 pagesDimethylpananthereneMD Redwan IslamNo ratings yet
- Bomb Calorimeter ReportDocument18 pagesBomb Calorimeter ReportRami Chaoul0% (1)
- Pharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GDocument11 pagesPharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GSujanNo ratings yet
- Conformational Analysis: Carey & Sundberg: Part A Chapter 3Document56 pagesConformational Analysis: Carey & Sundberg: Part A Chapter 3swastikNo ratings yet
- PracticeTests Answers All Chem350Document114 pagesPracticeTests Answers All Chem350Allan DNo ratings yet
- Chapter - 4 Alkanes and Cycloalkanes - HJMDocument78 pagesChapter - 4 Alkanes and Cycloalkanes - HJMMarisol AcostaNo ratings yet
- Rainer Herges - Topology in Chemistry: Designing Mobius MoleculesDocument23 pagesRainer Herges - Topology in Chemistry: Designing Mobius MoleculesOmsadsiNo ratings yet
- Organic Reactions 61Document215 pagesOrganic Reactions 61Pablo BarriosNo ratings yet
- BT8403-Enzyme Technology and Biotransformation Department of BiotechnologyDocument106 pagesBT8403-Enzyme Technology and Biotransformation Department of BiotechnologyAnupriyaNo ratings yet
- 235practice Exam 1Document11 pages235practice Exam 1Zia RathoreNo ratings yet
- IsomerismDocument61 pagesIsomerismSaket DubeyNo ratings yet
- Use of Cyclopropanes and Their Derivatives in Organic SynthesisDocument34 pagesUse of Cyclopropanes and Their Derivatives in Organic SynthesisDavid Lo100% (1)
- 2m Engl Isomerism 2021 For StudDocument96 pages2m Engl Isomerism 2021 For StudGhost ShooterNo ratings yet
- Cyclopentane SynthesisDocument19 pagesCyclopentane SynthesisCyrene MBolañosNo ratings yet
- Talk 11 Practical UmbrellaDocument56 pagesTalk 11 Practical UmbrellaAnna VeraNo ratings yet
- Organic Chemistry Some Basic Principles and TechniquesDocument34 pagesOrganic Chemistry Some Basic Principles and Techniquestannie2512No ratings yet
- 2016 Fall Midterm 1 OChem 1 KeyDocument20 pages2016 Fall Midterm 1 OChem 1 KeyAlex LeungNo ratings yet
- 5 Conformational AnalysisDocument42 pages5 Conformational AnalysisHASRHONNo ratings yet
- 61 NotesDocument133 pages61 NotesEman NoamanNo ratings yet
- Cyclic Alkanes & AlkenesDocument18 pagesCyclic Alkanes & AlkenesbgbscgvNo ratings yet
Untitled
Untitled
Uploaded by
RAVI52680 ratings0% found this document useful (0 votes)
9 views42 pagesLower cycloalkanes like cyclopropane and cyclobutane experience more angle strain due to deviations from the normal tetrahedral bond angle of 109.5°. This makes them more reactive and prone to ring-opening reactions like halogenation, hydrogenation, and reactions with hydrogen halides. Baeyer's strain theory proposed that angle strain increases with greater deviations from the optimal 109.5° bond angle and influences the relative stability and reactivity of cycloalkanes. Later theories such as bent bonds and puckered ring conformations provided alternative explanations for the reactivity of small ring cycloalkanes.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentLower cycloalkanes like cyclopropane and cyclobutane experience more angle strain due to deviations from the normal tetrahedral bond angle of 109.5°. This makes them more reactive and prone to ring-opening reactions like halogenation, hydrogenation, and reactions with hydrogen halides. Baeyer's strain theory proposed that angle strain increases with greater deviations from the optimal 109.5° bond angle and influences the relative stability and reactivity of cycloalkanes. Later theories such as bent bonds and puckered ring conformations provided alternative explanations for the reactivity of small ring cycloalkanes.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
9 views42 pagesUntitled
Untitled
Uploaded by
RAVI5268Lower cycloalkanes like cyclopropane and cyclobutane experience more angle strain due to deviations from the normal tetrahedral bond angle of 109.5°. This makes them more reactive and prone to ring-opening reactions like halogenation, hydrogenation, and reactions with hydrogen halides. Baeyer's strain theory proposed that angle strain increases with greater deviations from the optimal 109.5° bond angle and influences the relative stability and reactivity of cycloalkanes. Later theories such as bent bonds and puckered ring conformations provided alternative explanations for the reactivity of small ring cycloalkanes.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 42
Reactions:-
Just because unstability and due to
holding the character of angle strain
they (lower cycloalkane like
cyclopropane and cyclobutane) are
more suseptible to ring opening
reactions.
Halogenation – Photo-halogenation
Catalytic-halogenation
Catalytic Hydrogenation
Effect of Heat
Reaction with hydrogen halides
Halogenation – Photo-halogenation
Catalytic-halogenation
Catalytic Hydrogenation
Effect of Heat
Reaction with hydrogen halides
Baeyer’s Strain Theory
Baeyer’s theory is based upon some
assumptions that are helpful to understand
instability of cycloalkane ring systems.
All ring systems are planar. Deviation from
normal tetrahedral angles results in to
instable cycloalkanes.
The large ring systems involve negative strain
hence do not exists.
.
Adolf Baeyer (1885). Proposed a theory to explain the
relative stability of the first few cycloalkanes on the
fact that the normal angle between any pair of bonds
of carbon atom is 109⁰ 28' (or 109.5⁰) in tetrahedral
geometry (methane molecule).
Baeyer postulated that any deviation of bond angles
from the normal tetrahedral value would impose a
condition of internal strain on the ring. This is called
Angle Strain, which determined the stability of the
ring.
Baeyer proposed that the optimum overlap of atomic
orbitals is achieved for bond angel of 109.5⁰ (for
carbon atom, in a molecule if it is sp3 hybridized and
orbital overlap is maximum).
In short, it is ideal bond angle for alkane compounds
that produces maximum bond strength and stable
molecule.
Fig.1: Orbital overlap between (A) Propane (B) Cyclopropane.
Maximum overlap occurs in propane
If bond angles deviate from the ideal then the
ring produce strain. Higher strain produces
higher instability, increased reactivity and
increases heat of combustion. In simple higher
the deviation lesser the instability.
According to Baeyer, the relative order of
stability for some common cycloalkanes is as
under
Cyclopentane > Cyclobutane > Cyclopropane
Table 1: Angale strain of first few cycloalkanes
Compound Bond angle Deviation Angle strain
Cyclopropane 60⁰ 49.5⁰ 24.75⁰
Cyclobutane 90⁰ 19.5⁰ 9.75⁰
Cyclopentane 108⁰ 1.5⁰ 0.75⁰
Cyclohexane 120⁰ 10.5⁰ -5.27⁰
Limitations of Baeyer’s angle strain theory
Baeyer was not able to explain the effect of angle strain in
larger ring systems.
According to Baeyer Cyclopentane should be much stable
than cyclohexane but practically it is reversed.
Larger ring systems are not possible according to Baeyer as
they have negative strain but they exist and much stable.
Larger ring systems are not planar but wrinkled (puckered)
to eliminate angle strain.
Coulson-Moffitt Model or Concept of Maximum
Overlap of Carbon Orbitals (Bent bond/Banana
bond Theory)
A bent bond, also known as a banana bond, is a
type of covalent chemical bond with geometry
somewhat indicative of a banana.
The term itself is a general representation of
electron density or configuration resembling a
similar "bent" structure within small ring
molecules, such as cyclopropane (C3H6) or as a
representation of double or triple bonds within a
compound that is an alternative to the sigma and pi
bond model.
Note that sigma bond (strong bond) involves end-on-overlap
and pi bond involves side-long overlap. Bent bond is
intermediate between sigma and pi bonding. The overlap
being neither end-on nor side-long. This makes the overlap
less efficient than sigma overlap and the cyclopropane C-C
bonds weaker than normal C-C sigma bonds
Coulson-Moffits proposed that the smallest carbon
valency angle which can be 104⁰. Therefore,
suggested that in cyclopropane only a partial overlap
of the sp3 hybrid orbitals occurs, so C-C-C bond
angle is decreased slightly from 109.5⁰ to 104⁰ and
the H-C-H angle increased to 120⁰ compare to
normal tetrahedral angle.
• This decrease in overlap results in weakening of
the bond and therefore partially explains the
instability of cyclopropane.
• In case of cyclobutane, also has bent bonds but the
decrease in overlap is less than in the case of
cyclopropane.
• Cyclobutane is, therefore more stable (not
participate in ring-opening reactions) and less
reactive than cyclopropane.
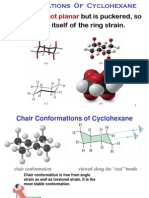
Sachse-Mohr Theory (Theory of Strainless Rings)
Sachse Mohr’s theory proposed that higher member
ring can become free from strain if all the ring carbons
are not forced into one plane. They exhibit in two non-
planar ‘folded’ or ‘puckered’ conformations both of
which are completely free from strain. These are
strainless as the carbon atoms lie in different planes
and the normal valency angle (109.5⁰) is retained.
These are called the ‘Chair’ Form or the ‘Z’ Form
and the ‘Boat’ Form or the ‘C’ Form because of their
shapes.
The chair conformation is the most stable
conformation of cyclohexane.
In chair cyclohexane there are two types of positions,
axial and equatorial. The axial positions point
perpendicular to the plane of the ring, whereas the
equatorial positions are around the plane of the ring.
Mohr (1918) further elaborated this theory and
applied it to compounds with the two rings fused
together.
You might also like
- Unit 5Document42 pagesUnit 5RAVI5268No ratings yet
- Cycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheoryDocument5 pagesCycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheorySayed AltamashNo ratings yet
- Guru Nanak Institute of Pharmaceutical Science and TechnologyDocument13 pagesGuru Nanak Institute of Pharmaceutical Science and TechnologySomali SenguptaNo ratings yet
- Pharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GDocument11 pagesPharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GSujanNo ratings yet
- Baeyrs Strain TheoryDocument5 pagesBaeyrs Strain TheorySk ZNo ratings yet
- Relative Stabilities of CycloakanesDocument8 pagesRelative Stabilities of CycloakanesKamal KishoreNo ratings yet
- What Are Cyclo-Alkanes?Document6 pagesWhat Are Cyclo-Alkanes?gfgfccNo ratings yet
- Cyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Document6 pagesCyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Tushar GiradkarNo ratings yet
- Cyclic Aliphatic Compounds: NomenclatureDocument19 pagesCyclic Aliphatic Compounds: NomenclatureWinnie SantiagoNo ratings yet
- Tutorial07b OrganicCycloalkanesDocument24 pagesTutorial07b OrganicCycloalkanesHana NisrinaNo ratings yet
- Chemistry PDFDocument9 pagesChemistry PDFRájésh KùmârNo ratings yet
- Cyclo AlkanesDocument38 pagesCyclo Alkanesdinesh111180No ratings yet
- Cyclo Al KanesDocument3 pagesCyclo Al KanesIbraim ZekirNo ratings yet
- LecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryDocument8 pagesLecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryKevalNo ratings yet
- Coulson and MoffittDocument2 pagesCoulson and MoffittTanya 60No ratings yet
- Lesson StructureDocument19 pagesLesson Structurer karthickNo ratings yet
- 3.4 The Shapes of Cycloalkanes: Planar or Nonplanar?Document29 pages3.4 The Shapes of Cycloalkanes: Planar or Nonplanar?juanNo ratings yet
- Cyclic Alkanes & AlkenesDocument18 pagesCyclic Alkanes & AlkenesbgbscgvNo ratings yet
- Conformational Analysis of CyclopentaneDocument11 pagesConformational Analysis of CyclopentaneNimra MalikNo ratings yet
- Conformational Analysis For StudentsDocument89 pagesConformational Analysis For Studentsindrapal kumarNo ratings yet
- SCH 102 Lecture Week 8 2018-9Document27 pagesSCH 102 Lecture Week 8 2018-9Radhika TopwalNo ratings yet
- Chapter 4Document33 pagesChapter 4채종희No ratings yet
- MODULE 5: Conformational Analysis of Alkanes and CyclohexanesDocument8 pagesMODULE 5: Conformational Analysis of Alkanes and CyclohexanesARMAN AKRAM BIN OMAR / UPMNo ratings yet
- Conformational AnalysisDocument10 pagesConformational AnalysisPG Chemistry PG ChemistryNo ratings yet
- Conformation AnalysisDocument41 pagesConformation AnalysisJoseNo ratings yet
- Angle Strain Torsional Strain Ring StrainDocument6 pagesAngle Strain Torsional Strain Ring StrainchuasioklengNo ratings yet
- InorganicDocument43 pagesInorganicShobhit ZakhmiNo ratings yet
- Chapter 4. Alkanes (BSChem)Document59 pagesChapter 4. Alkanes (BSChem)Chase PattersonNo ratings yet
- Chapter 4 With Video LinksDocument37 pagesChapter 4 With Video LinksDoom RefugeNo ratings yet
- Organic Chemistry: M. R. Naimi-JamalDocument81 pagesOrganic Chemistry: M. R. Naimi-JamalHamidatun NisaNo ratings yet
- Reaction MechanismDocument30 pagesReaction Mechanismantoniostark0010No ratings yet
- 1515564149CHE P1 M16 EtextDocument22 pages1515564149CHE P1 M16 EtextElangovan NatarajanNo ratings yet
- GeneralChem LS 25 PDFDocument25 pagesGeneralChem LS 25 PDFSunil NahataNo ratings yet
- UntitledDocument46 pagesUntitled양우경No ratings yet
- Lecture 31 PDFDocument17 pagesLecture 31 PDFRachit ShahNo ratings yet
- DecalinsDocument25 pagesDecalinstessyNo ratings yet
- Conformation & Conformational IsomersDocument4 pagesConformation & Conformational Isomerspulkit asatiNo ratings yet
- CHEM 210-1 Organic Chemistry NotesDocument26 pagesCHEM 210-1 Organic Chemistry NotesRobert GardnerNo ratings yet
- Alkanes: Sakti Hidayati FikriyaDocument26 pagesAlkanes: Sakti Hidayati FikriyaShinta Novita SariNo ratings yet
- Cyclohexane Conformational AnalysisDocument1 pageCyclohexane Conformational Analysis휘승No ratings yet
- Stereo KIMIA, KIMIA ORGANIK 2Document92 pagesStereo KIMIA, KIMIA ORGANIK 2Mutia HadiyantiNo ratings yet
- Chapter 3Document27 pagesChapter 3Siddarth PalletiNo ratings yet
- Alkanes, Alkenes, Alkynes, and CycloalkanesDocument162 pagesAlkanes, Alkenes, Alkynes, and CycloalkanesNurtasha AtikahNo ratings yet
- Carbocations 2Document16 pagesCarbocations 2Surabhi BansalNo ratings yet
- Monocyclic DisubstitutedDocument3 pagesMonocyclic DisubstitutedPs SatchithNo ratings yet
- محاضرات العضوية-1-الثالثةDocument13 pagesمحاضرات العضوية-1-الثالثةlindanenkosiNo ratings yet
- Chapter 3: Structure and Stereochemistry of AlkanesDocument42 pagesChapter 3: Structure and Stereochemistry of AlkanesKera Maria AllengerNo ratings yet
- StudentDocument31 pagesStudentnanaNo ratings yet
- Organic - Class 8Document41 pagesOrganic - Class 8Sajan Singh LUCKYNo ratings yet
- Cyclic AlkanesDocument35 pagesCyclic AlkanesKunjal100% (2)
- Conformaciones Diferentes Configuraciones DiferentesDocument45 pagesConformaciones Diferentes Configuraciones DiferentesJuan CarlosNo ratings yet
- Eme104 Lecture 2Document8 pagesEme104 Lecture 2grantwekesaNo ratings yet
- Conformational IsomersDocument8 pagesConformational Isomersshirodkar_16593No ratings yet
- BSES Topic 06 Cycloalkanes and Aromatic CompoundsDocument42 pagesBSES Topic 06 Cycloalkanes and Aromatic CompoundsJhunessa Mae JalagatNo ratings yet
- Organic Chemistry TermsDocument7 pagesOrganic Chemistry TermssbelodoNo ratings yet
- Conformational Analysis:: Chapter - 6 Stereochemistry NotesDocument12 pagesConformational Analysis:: Chapter - 6 Stereochemistry NotesMohit KambojNo ratings yet
- Organic Chemistry TutorialsDocument11 pagesOrganic Chemistry TutorialsSuyash OnkarNo ratings yet
- Conformations IIDocument16 pagesConformations IIsndNo ratings yet
- U-4, Poc-I, Carewell PharmaDocument19 pagesU-4, Poc-I, Carewell PharmaRAVI5268No ratings yet
- Unit 5Document42 pagesUnit 5RAVI5268No ratings yet
- UJPBS 23305 (RS)Document6 pagesUJPBS 23305 (RS)RAVI5268No ratings yet
- POC I Practical Carewell PharmaDocument27 pagesPOC I Practical Carewell PharmaRAVI5268No ratings yet
- ChatLog Online Workshop On Child Rights and Protection For Coordinators - Officers of National Service Scheme - NSS - Date - 19 - 20 August2020 - Timings 3 - 00PM - 5 - 00PM - 2020-08-19 16 - 19Document2 pagesChatLog Online Workshop On Child Rights and Protection For Coordinators - Officers of National Service Scheme - NSS - Date - 19 - 20 August2020 - Timings 3 - 00PM - 5 - 00PM - 2020-08-19 16 - 19RAVI5268No ratings yet
- E HealthDocument57 pagesE HealthRAVI5268100% (2)
- DTMM and COSMIC Molecular Mechanics Parameters For AlkylsilanesDocument14 pagesDTMM and COSMIC Molecular Mechanics Parameters For AlkylsilanesLuu Xuan CuongNo ratings yet
- Neral Organic Chemistry (62-80)Document19 pagesNeral Organic Chemistry (62-80)udaysrinivasNo ratings yet
- Important Concepts: Conformational Analysis of AlkanesDocument4 pagesImportant Concepts: Conformational Analysis of AlkanesKayla JoezetteNo ratings yet
- Conformational AnalysisDocument53 pagesConformational AnalysisDavidAlejoNo ratings yet
- CH 4 AlkanesDocument16 pagesCH 4 AlkanesBrittnay Marie100% (4)
- Stereochemistry of Organic CompoundsDocument31 pagesStereochemistry of Organic CompoundsSrinivasulu KonetiNo ratings yet
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Document8 pagesChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNo ratings yet
- Chem 210 PSU Exam 1Document7 pagesChem 210 PSU Exam 1milkah mwauraNo ratings yet
- StereochemistryDocument108 pagesStereochemistryAllan DNo ratings yet
- Fiitjee Test PaperDocument23 pagesFiitjee Test PaperNikita GargNo ratings yet
- DimethylpananthereneDocument11 pagesDimethylpananthereneMD Redwan IslamNo ratings yet
- Bomb Calorimeter ReportDocument18 pagesBomb Calorimeter ReportRami Chaoul0% (1)
- Pharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GDocument11 pagesPharmaceutical Organic Chemistry-I Bayer Strain Theory: Satheesh Kumar GSujanNo ratings yet
- Conformational Analysis: Carey & Sundberg: Part A Chapter 3Document56 pagesConformational Analysis: Carey & Sundberg: Part A Chapter 3swastikNo ratings yet
- PracticeTests Answers All Chem350Document114 pagesPracticeTests Answers All Chem350Allan DNo ratings yet
- Chapter - 4 Alkanes and Cycloalkanes - HJMDocument78 pagesChapter - 4 Alkanes and Cycloalkanes - HJMMarisol AcostaNo ratings yet
- Rainer Herges - Topology in Chemistry: Designing Mobius MoleculesDocument23 pagesRainer Herges - Topology in Chemistry: Designing Mobius MoleculesOmsadsiNo ratings yet
- Organic Reactions 61Document215 pagesOrganic Reactions 61Pablo BarriosNo ratings yet
- BT8403-Enzyme Technology and Biotransformation Department of BiotechnologyDocument106 pagesBT8403-Enzyme Technology and Biotransformation Department of BiotechnologyAnupriyaNo ratings yet
- 235practice Exam 1Document11 pages235practice Exam 1Zia RathoreNo ratings yet
- IsomerismDocument61 pagesIsomerismSaket DubeyNo ratings yet
- Use of Cyclopropanes and Their Derivatives in Organic SynthesisDocument34 pagesUse of Cyclopropanes and Their Derivatives in Organic SynthesisDavid Lo100% (1)
- 2m Engl Isomerism 2021 For StudDocument96 pages2m Engl Isomerism 2021 For StudGhost ShooterNo ratings yet
- Cyclopentane SynthesisDocument19 pagesCyclopentane SynthesisCyrene MBolañosNo ratings yet
- Talk 11 Practical UmbrellaDocument56 pagesTalk 11 Practical UmbrellaAnna VeraNo ratings yet
- Organic Chemistry Some Basic Principles and TechniquesDocument34 pagesOrganic Chemistry Some Basic Principles and Techniquestannie2512No ratings yet
- 2016 Fall Midterm 1 OChem 1 KeyDocument20 pages2016 Fall Midterm 1 OChem 1 KeyAlex LeungNo ratings yet
- 5 Conformational AnalysisDocument42 pages5 Conformational AnalysisHASRHONNo ratings yet
- 61 NotesDocument133 pages61 NotesEman NoamanNo ratings yet
- Cyclic Alkanes & AlkenesDocument18 pagesCyclic Alkanes & AlkenesbgbscgvNo ratings yet