Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
1 viewsThermo Presentation
Thermo Presentation
Uploaded by
Muhammad AbtaheeElectrochemistry is the branch of chemistry dealing with the interconversion of chemical and electrical energy. It involves the study of redox reactions where electrons are transferred from one substance to another. Oxidation is the loss of electrons while reduction is the gain of electrons. Oxidizing agents remove electrons while reducing agents donate electrons. The electromotive force (EMF) of an electrochemical cell is related to the Gibbs free energy change of the cell reactions. Thermodynamic properties such as entropy and enthalpy changes can be determined from electrochemical measurements by varying temperature. Metals are arranged in an electrochemical series based on their electrode potentials. Pourbaix diagrams show the conditions under which metals will be immune, passive,
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You might also like
- Practical Refraction EnglishDocument56 pagesPractical Refraction Englisherin100% (3)
- 1 Thermochemistry (Semester 2)Document32 pages1 Thermochemistry (Semester 2)Esther NgiengNo ratings yet
- Cells and Batteries Revised NotesDocument28 pagesCells and Batteries Revised NotesRoynika shaluNo ratings yet
- Electrochemical Energy: Unit 2. Matter and Energy: CDocument43 pagesElectrochemical Energy: Unit 2. Matter and Energy: CMayNo ratings yet
- Electrochemistry: Neet - Chem-Electro-KerthanaDocument12 pagesElectrochemistry: Neet - Chem-Electro-KerthanaManickam GnanashekaranNo ratings yet
- ElectrochemistryDocument17 pagesElectrochemistryAbhianv GuptaNo ratings yet
- Corrosionscienceroshni 180423175407Document26 pagesCorrosionscienceroshni 180423175407MohankumarNo ratings yet
- Conductors: NCERT Notes For Class 12 Chemistry Chapter 3: ElectrochemistryDocument12 pagesConductors: NCERT Notes For Class 12 Chemistry Chapter 3: ElectrochemistryDev Printing SolutionNo ratings yet
- Chemistry Notes For Class 12 Chapter 3 ElectrochemistryDocument17 pagesChemistry Notes For Class 12 Chapter 3 ElectrochemistryRavi Kumar50% (4)
- Hitesh Rathod 201me140Document8 pagesHitesh Rathod 201me140Pavan KumarNo ratings yet
- Electochemistry PDFDocument29 pagesElectochemistry PDFAnshu KarmacharyaNo ratings yet
- Module - 1: Engineering Chemistry 18che12/22Document16 pagesModule - 1: Engineering Chemistry 18che12/22darshansraichur12No ratings yet
- DR - MPR - ElectrochemistryDocument25 pagesDR - MPR - ElectrochemistryRishan KundetyNo ratings yet
- Notes Chem NewDocument17 pagesNotes Chem Newilias1973No ratings yet
- Engineering Chemistry Module 1Document35 pagesEngineering Chemistry Module 1Audrey MendozaNo ratings yet
- MODULE 2 ElectrochemistryDocument31 pagesMODULE 2 ElectrochemistryChristian Mark De JesusNo ratings yet
- 22CHE22Document52 pages22CHE22GAT LIBRARYNo ratings yet
- Electro Chemistry SND CorrosionDocument10 pagesElectro Chemistry SND CorrosiondrgviswaNo ratings yet
- Unit-2 Battery TechnologyDocument47 pagesUnit-2 Battery TechnologyYash WaghmareNo ratings yet
- Electrochemical SeriesDocument9 pagesElectrochemical Seriesasim zeeshanNo ratings yet
- Peta ElektrolisisDocument10 pagesPeta ElektrolisisIqbal BlakeNo ratings yet
- Principles of CorrosionDocument15 pagesPrinciples of CorrosionwinarnobNo ratings yet
- 03.thermodynamics in Corrosion EngineeringDocument33 pages03.thermodynamics in Corrosion EngineeringAsad AlfautoreNo ratings yet
- ElectrochemistryDocument20 pagesElectrochemistry나야No ratings yet
- SESSION (2021-22) Class-Xii (Science) SUB-Chemistry CHAPTER-Electrochemistry Notes, Activities and Assignments (2021)Document7 pagesSESSION (2021-22) Class-Xii (Science) SUB-Chemistry CHAPTER-Electrochemistry Notes, Activities and Assignments (2021)Ashok KumarNo ratings yet
- Nernst Equation and Pourbaix Diagrams: Introduction and BackgroundDocument8 pagesNernst Equation and Pourbaix Diagrams: Introduction and BackgroundGrant HosieNo ratings yet
- Electrochemistry-12 2 11Document49 pagesElectrochemistry-12 2 11Vic VickyNo ratings yet
- Fundmentals Corrosion CH 2Document44 pagesFundmentals Corrosion CH 2عاشق الصمتNo ratings yet
- 1 Fundamentals of ElectrochemistryDocument74 pages1 Fundamentals of ElectrochemistryBalakrishnan Pedda GovindierNo ratings yet
- Electro Chemistry and CorrosionDocument10 pagesElectro Chemistry and CorrosiondeeparamaniNo ratings yet
- Module2-S9-13-Corrosion-Pourbaix DiagramDocument41 pagesModule2-S9-13-Corrosion-Pourbaix Diagramsundar57567No ratings yet
- Lecture Notes - Engineering ChemistryDocument56 pagesLecture Notes - Engineering ChemistryadamjosephNo ratings yet
- Electrochemistry Syllabus: Sengunthar Engineering College Department of ChemistryDocument10 pagesElectrochemistry Syllabus: Sengunthar Engineering College Department of ChemistrydeeparamaniNo ratings yet
- BasicsDocument38 pagesBasicsSwathi DineshNo ratings yet
- Electrochemistry and Energy Storage Systems Module 1 Notes 2018Document24 pagesElectrochemistry and Energy Storage Systems Module 1 Notes 2018Mohith BC100% (1)
- Electrochemical Series PDFDocument7 pagesElectrochemical Series PDFJayashree SNo ratings yet
- Electrical Energy and Vice Versa. It Is Basically The Study ofDocument11 pagesElectrical Energy and Vice Versa. It Is Basically The Study ofJake WooNo ratings yet
- Electrochemistry and Corrosion: Question & Answers:: 1.Q. Derive Nernst Equation and Give ApplicationsDocument5 pagesElectrochemistry and Corrosion: Question & Answers:: 1.Q. Derive Nernst Equation and Give ApplicationsSai shravya GorekarNo ratings yet
- ElectrochemistryDocument26 pagesElectrochemistryJasbir SinghNo ratings yet
- Chemistry 3Document7 pagesChemistry 3syed waseemNo ratings yet
- Chapter 3 Electro ChemistryDocument20 pagesChapter 3 Electro ChemistryKritika MishraNo ratings yet
- Electrochemistry Part 1Document35 pagesElectrochemistry Part 1ABHINAVNo ratings yet
- Electrolytic ProcessesDocument9 pagesElectrolytic ProcessesIshitha ChauhanNo ratings yet
- Corrosion MechanismsDocument64 pagesCorrosion MechanismsRahul PandeyNo ratings yet
- Iitm Diploma 1st Year Applied ChemistryDocument35 pagesIitm Diploma 1st Year Applied Chemistryneetugkp6388No ratings yet
- Unit 3 ElectrochemistryDocument14 pagesUnit 3 ElectrochemistrySuresh Dasaraddi100% (1)
- Electrochemistry Short NotesDocument5 pagesElectrochemistry Short Notestukutanigel423No ratings yet
- ElectrochemistryDocument2 pagesElectrochemistryria sNo ratings yet
- ElectroDocument13 pagesElectrodulalsushant3No ratings yet
- Concept of ElectrochemitryDocument15 pagesConcept of ElectrochemitryKritika SainiNo ratings yet
- Introduction To ElectrochemistryDocument40 pagesIntroduction To ElectrochemistryAngates1100% (2)
- ElectrochemistryDocument14 pagesElectrochemistryAMEEDA JABEENNo ratings yet
- Ec Notes - 11-7-18Document82 pagesEc Notes - 11-7-18Yasir AliNo ratings yet
- Electrochemical Cells: Electrode PotentialDocument20 pagesElectrochemical Cells: Electrode PotentialMunazNo ratings yet
- NEET Electrochemistry FinalDocument204 pagesNEET Electrochemistry Finaljaisree291006No ratings yet
- Chapter 04: Electrochemistry: Sheet-1Document35 pagesChapter 04: Electrochemistry: Sheet-1imtial kashemNo ratings yet
- 1219 159 578 Module 1Document86 pages1219 159 578 Module 1Mercy SajiNo ratings yet
- 2nd PhysicalDocument7 pages2nd Physicalsmarialatif11No ratings yet
- Section-A (Electro Chemistry) : A Brief Review of The Basic ConceptsDocument33 pagesSection-A (Electro Chemistry) : A Brief Review of The Basic ConceptsKanchanNo ratings yet
- Corrosion 4Document34 pagesCorrosion 4Faria Sultana MimiNo ratings yet
- Module 2 Notes Chem Mescenotes - inDocument31 pagesModule 2 Notes Chem Mescenotes - inHafizNo ratings yet
- A 197 - A 197M - 00 (2015)Document4 pagesA 197 - A 197M - 00 (2015)phaindikaNo ratings yet
- PHOTOVOLTAICSDocument27 pagesPHOTOVOLTAICSbrian otienoNo ratings yet
- CHT307 - Ktu QbankDocument3 pagesCHT307 - Ktu QbankYuxin CasioNo ratings yet
- Materials: Ffect of Different Compatibilizers On The Properties /poly (Butylene Adipate-CoDocument16 pagesMaterials: Ffect of Different Compatibilizers On The Properties /poly (Butylene Adipate-CoSiddharthBhasneyNo ratings yet
- 3G3mx2-v2 Ds e 1 1 csm1126864Document54 pages3G3mx2-v2 Ds e 1 1 csm1126864Pertti HänninenNo ratings yet
- Study of An Aquifer in A Semi-Arid Area Using MRS, FDEM, TDEM and ERT Methods (Youssoufia and Khouribga, Morocco)Document4 pagesStudy of An Aquifer in A Semi-Arid Area Using MRS, FDEM, TDEM and ERT Methods (Youssoufia and Khouribga, Morocco)tikroumineNo ratings yet
- Flow ChartDocument2 pagesFlow ChartJames PerriamNo ratings yet
- Module 2 Laplace TransformsDocument36 pagesModule 2 Laplace TransformsErnie Mark Patosa MaratasNo ratings yet
- Linear Motion Power Transmission Design Guide 02Document274 pagesLinear Motion Power Transmission Design Guide 02Ravindra KirangeNo ratings yet
- Schedule - JEE Advanced Crash Course 2024Document2 pagesSchedule - JEE Advanced Crash Course 2024saurav.saha1984No ratings yet
- IISER Syllabus: MathematicsDocument3 pagesIISER Syllabus: MathematicsIIT aspNo ratings yet
- Material List For PLC ProjectsDocument1 pageMaterial List For PLC ProjectsEngr Muhib KhÅñNo ratings yet
- Me6411 Manufacturing Technology-II Lab ManualDocument35 pagesMe6411 Manufacturing Technology-II Lab ManualdibyenindusNo ratings yet
- CM-PFS: Three-Phase Monitoring RelaysDocument10 pagesCM-PFS: Three-Phase Monitoring RelaysfirozNo ratings yet
- (NS) XII EM One Word Vol - IDocument12 pages(NS) XII EM One Word Vol - IAnishaNo ratings yet
- Is It Simple To Explain Simple Experiments The Heavy Newspaper' Stick BreakDocument6 pagesIs It Simple To Explain Simple Experiments The Heavy Newspaper' Stick Break이온유No ratings yet
- Anaytic Questioner - FinalDocument12 pagesAnaytic Questioner - FinalEdwin LoquinaNo ratings yet
- O Level Physics Text BookDocument111 pagesO Level Physics Text BookMatabaro BenjaminNo ratings yet
- Design of Helical SpringDocument11 pagesDesign of Helical SpringNarender Kumar100% (1)
- Lesson 10 - Haloalkanes & HaloarenesDocument170 pagesLesson 10 - Haloalkanes & HaloarenesAwez FahadNo ratings yet
- Module 4 Work Energy PowerDocument7 pagesModule 4 Work Energy PowerGreen BrainNo ratings yet
- Silicon Institute of Technology Department of Electrical & Electronics Engineering Quiz Test Electrical Machine-II Lab 5 Sem EEE-B, Gr.-I & IIDocument3 pagesSilicon Institute of Technology Department of Electrical & Electronics Engineering Quiz Test Electrical Machine-II Lab 5 Sem EEE-B, Gr.-I & IIT. VARMANo ratings yet
- EE6512-Electrical Machines LaboratoryDocument82 pagesEE6512-Electrical Machines LaboratoryGopinath B L NaiduNo ratings yet
- Write Answers To All NCERT Intext Solved & Unsolved Problems. 2. Write Answers To All NCERT Questions in ExercisesDocument2 pagesWrite Answers To All NCERT Intext Solved & Unsolved Problems. 2. Write Answers To All NCERT Questions in ExercisesJagriti DaryaniNo ratings yet
- Thermal ComfortDocument4 pagesThermal ComfortVidya HittiNo ratings yet
- Lecture - 8: Fluid Motion: Streamlines EtcDocument13 pagesLecture - 8: Fluid Motion: Streamlines Etcrohit singhNo ratings yet
- CSE 28363 - Structural Concrete Design: Lecture 10: Shear and ServiceabilityDocument44 pagesCSE 28363 - Structural Concrete Design: Lecture 10: Shear and ServiceabilityBitch CarrieNo ratings yet
- Temp4 Design ProceduresDocument23 pagesTemp4 Design ProceduresAhmed AdelNo ratings yet
Thermo Presentation
Thermo Presentation
Uploaded by
Muhammad Abtahee0 ratings0% found this document useful (0 votes)
1 views14 pagesElectrochemistry is the branch of chemistry dealing with the interconversion of chemical and electrical energy. It involves the study of redox reactions where electrons are transferred from one substance to another. Oxidation is the loss of electrons while reduction is the gain of electrons. Oxidizing agents remove electrons while reducing agents donate electrons. The electromotive force (EMF) of an electrochemical cell is related to the Gibbs free energy change of the cell reactions. Thermodynamic properties such as entropy and enthalpy changes can be determined from electrochemical measurements by varying temperature. Metals are arranged in an electrochemical series based on their electrode potentials. Pourbaix diagrams show the conditions under which metals will be immune, passive,
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentElectrochemistry is the branch of chemistry dealing with the interconversion of chemical and electrical energy. It involves the study of redox reactions where electrons are transferred from one substance to another. Oxidation is the loss of electrons while reduction is the gain of electrons. Oxidizing agents remove electrons while reducing agents donate electrons. The electromotive force (EMF) of an electrochemical cell is related to the Gibbs free energy change of the cell reactions. Thermodynamic properties such as entropy and enthalpy changes can be determined from electrochemical measurements by varying temperature. Metals are arranged in an electrochemical series based on their electrode potentials. Pourbaix diagrams show the conditions under which metals will be immune, passive,
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
1 views14 pagesThermo Presentation
Thermo Presentation
Uploaded by
Muhammad AbtaheeElectrochemistry is the branch of chemistry dealing with the interconversion of chemical and electrical energy. It involves the study of redox reactions where electrons are transferred from one substance to another. Oxidation is the loss of electrons while reduction is the gain of electrons. Oxidizing agents remove electrons while reducing agents donate electrons. The electromotive force (EMF) of an electrochemical cell is related to the Gibbs free energy change of the cell reactions. Thermodynamic properties such as entropy and enthalpy changes can be determined from electrochemical measurements by varying temperature. Metals are arranged in an electrochemical series based on their electrode potentials. Pourbaix diagrams show the conditions under which metals will be immune, passive,
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 14
Electrochemistry
and its uses
Muhammad Abtahee 32
Abdullah Kiani 72
Introduction
The branch of chemistry that deals with the chemical
changes produced by electricity and the production of
electricity by chemical changes.
It is the study of interconversion of chemical energy and
electrical energy.
Study of redox reaction(transfer of electrons from one
substance to another).
Oxidation and Reduction
It is the loss of electrons during a reaction by a molecule,
atom or ion.
Oxidation occurs when the oxidation state of a molecule,
atom or ion is increased.
Reduction occurs when there is a gain of electrons or the
oxidation state of an atom, molecule, or ion decreases.
Example:
H2 → 2 H+ + 2 e-
F2 + 2 e- → 2 F-
H2 + F2 → 2 HF
Reducing and oxidizing agent
Oxidizing agents removes electrons from another
substance in a redox reaction.
Reducing agents donates electrons to another substance in
a redox reaction.
Example:
Cu(s)+2 Ag+(aq) →Cu2+(aq)+2 Ag(s).
EMF and its relation with Gibbs
free energy ΔG
Energy per unit electric charge that is imparted by an
energy source, such as an electric generator or a battery.
Relation:
Gibbs free energy is the change in the amount of energy
available in a chemical system to do work.
In an electrochemical cell, the work done is dependent on
the number of coulombs of charge transferred and the
energy available.
ΔG = –zFE
Entropy Change
Entropy is the measure of disorder.
Electrochemical measurements are easy and quick to
obtain thermodynamic data.
-ΔS= ΔG/ΔT
Enthalpy Change
Enthalpy is the total heat content of the system.
ΔH= ΔG + TΔS
To find ΔG, ΔH, ΔS :
Set up an appropriate electrochemical cell
Measure EMF at various temperatures (3 or 4
temperatures).
EMF Series
An electromotive force series is a metal's ranking in
respect to inherent reactivity.
All metals have arranged in a series according to their
standard potential values.
The more positive value corresponds to noble metals and
the more negative value to more reactive metals.
If two metals make up a cell , the more active metal acts as
the anode and the more noble metal of the two will act as
the cathode.
Electrochemical Series
A serial arrangement of metallic elements or ions
according to their electrode potentials determined under
specified conditions.
Metals are ranked in accordance with their potential in 1 N
solution of their solutions.
Hydrogen is zero reference.
If metal has positive value it is called noble metal or semi
noble such as silver, copper etc.
If metal has negative value it is called active metal such as
iron, aluminum, magnesium and zinc.
Limitations of EMF series
In real solutions, activities of metal ions in equilibrium with the
respective metals usually do not equal to unity.
The position of a metal in EMF series with respect to another
metal may change because of complex formation.
Alloys are not included in EMF series.
In oxidizing environment, some metals undergo passivation.
Transition metals usually shows passive behavior in aerated
aqueous environment. This dual position of some metals is not
reflected in EMF series.
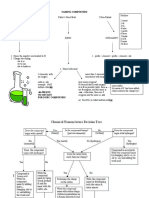
Pourbaix Diagram
Pourbaix diagrams shows conditions of potential and pH under
which a metal either be:
Immune.
Passive.
Corrode.
If hydrogen ions are involved we will get pH.
If electrons are involved we will get potential.
Horizontal lines mean constant potential.
Vertical lines mean constant pH.
Inclined lines will have both pH and potential.
Pourbaix Diagram(Cont.)
Uses of Electrochemistry
Electrochemistry can be useful in extraction, refining, plating,
corrosion and its control, power generation, melt composition,
thermo data etc.
Electrolytic cells are used in the electro refining of many non-
ferrous metals. They are also used in the electro winning of these
metals.
Electrochemistry is important for creating new technologies that
are ecofriendly.
It bring about non-spontaneous chemical transformations.
Thank You
You might also like
- Practical Refraction EnglishDocument56 pagesPractical Refraction Englisherin100% (3)
- 1 Thermochemistry (Semester 2)Document32 pages1 Thermochemistry (Semester 2)Esther NgiengNo ratings yet
- Cells and Batteries Revised NotesDocument28 pagesCells and Batteries Revised NotesRoynika shaluNo ratings yet
- Electrochemical Energy: Unit 2. Matter and Energy: CDocument43 pagesElectrochemical Energy: Unit 2. Matter and Energy: CMayNo ratings yet
- Electrochemistry: Neet - Chem-Electro-KerthanaDocument12 pagesElectrochemistry: Neet - Chem-Electro-KerthanaManickam GnanashekaranNo ratings yet
- ElectrochemistryDocument17 pagesElectrochemistryAbhianv GuptaNo ratings yet
- Corrosionscienceroshni 180423175407Document26 pagesCorrosionscienceroshni 180423175407MohankumarNo ratings yet
- Conductors: NCERT Notes For Class 12 Chemistry Chapter 3: ElectrochemistryDocument12 pagesConductors: NCERT Notes For Class 12 Chemistry Chapter 3: ElectrochemistryDev Printing SolutionNo ratings yet
- Chemistry Notes For Class 12 Chapter 3 ElectrochemistryDocument17 pagesChemistry Notes For Class 12 Chapter 3 ElectrochemistryRavi Kumar50% (4)
- Hitesh Rathod 201me140Document8 pagesHitesh Rathod 201me140Pavan KumarNo ratings yet
- Electochemistry PDFDocument29 pagesElectochemistry PDFAnshu KarmacharyaNo ratings yet
- Module - 1: Engineering Chemistry 18che12/22Document16 pagesModule - 1: Engineering Chemistry 18che12/22darshansraichur12No ratings yet
- DR - MPR - ElectrochemistryDocument25 pagesDR - MPR - ElectrochemistryRishan KundetyNo ratings yet
- Notes Chem NewDocument17 pagesNotes Chem Newilias1973No ratings yet
- Engineering Chemistry Module 1Document35 pagesEngineering Chemistry Module 1Audrey MendozaNo ratings yet
- MODULE 2 ElectrochemistryDocument31 pagesMODULE 2 ElectrochemistryChristian Mark De JesusNo ratings yet
- 22CHE22Document52 pages22CHE22GAT LIBRARYNo ratings yet
- Electro Chemistry SND CorrosionDocument10 pagesElectro Chemistry SND CorrosiondrgviswaNo ratings yet
- Unit-2 Battery TechnologyDocument47 pagesUnit-2 Battery TechnologyYash WaghmareNo ratings yet
- Electrochemical SeriesDocument9 pagesElectrochemical Seriesasim zeeshanNo ratings yet
- Peta ElektrolisisDocument10 pagesPeta ElektrolisisIqbal BlakeNo ratings yet
- Principles of CorrosionDocument15 pagesPrinciples of CorrosionwinarnobNo ratings yet
- 03.thermodynamics in Corrosion EngineeringDocument33 pages03.thermodynamics in Corrosion EngineeringAsad AlfautoreNo ratings yet
- ElectrochemistryDocument20 pagesElectrochemistry나야No ratings yet
- SESSION (2021-22) Class-Xii (Science) SUB-Chemistry CHAPTER-Electrochemistry Notes, Activities and Assignments (2021)Document7 pagesSESSION (2021-22) Class-Xii (Science) SUB-Chemistry CHAPTER-Electrochemistry Notes, Activities and Assignments (2021)Ashok KumarNo ratings yet
- Nernst Equation and Pourbaix Diagrams: Introduction and BackgroundDocument8 pagesNernst Equation and Pourbaix Diagrams: Introduction and BackgroundGrant HosieNo ratings yet
- Electrochemistry-12 2 11Document49 pagesElectrochemistry-12 2 11Vic VickyNo ratings yet
- Fundmentals Corrosion CH 2Document44 pagesFundmentals Corrosion CH 2عاشق الصمتNo ratings yet
- 1 Fundamentals of ElectrochemistryDocument74 pages1 Fundamentals of ElectrochemistryBalakrishnan Pedda GovindierNo ratings yet
- Electro Chemistry and CorrosionDocument10 pagesElectro Chemistry and CorrosiondeeparamaniNo ratings yet
- Module2-S9-13-Corrosion-Pourbaix DiagramDocument41 pagesModule2-S9-13-Corrosion-Pourbaix Diagramsundar57567No ratings yet
- Lecture Notes - Engineering ChemistryDocument56 pagesLecture Notes - Engineering ChemistryadamjosephNo ratings yet
- Electrochemistry Syllabus: Sengunthar Engineering College Department of ChemistryDocument10 pagesElectrochemistry Syllabus: Sengunthar Engineering College Department of ChemistrydeeparamaniNo ratings yet
- BasicsDocument38 pagesBasicsSwathi DineshNo ratings yet
- Electrochemistry and Energy Storage Systems Module 1 Notes 2018Document24 pagesElectrochemistry and Energy Storage Systems Module 1 Notes 2018Mohith BC100% (1)
- Electrochemical Series PDFDocument7 pagesElectrochemical Series PDFJayashree SNo ratings yet
- Electrical Energy and Vice Versa. It Is Basically The Study ofDocument11 pagesElectrical Energy and Vice Versa. It Is Basically The Study ofJake WooNo ratings yet
- Electrochemistry and Corrosion: Question & Answers:: 1.Q. Derive Nernst Equation and Give ApplicationsDocument5 pagesElectrochemistry and Corrosion: Question & Answers:: 1.Q. Derive Nernst Equation and Give ApplicationsSai shravya GorekarNo ratings yet
- ElectrochemistryDocument26 pagesElectrochemistryJasbir SinghNo ratings yet
- Chemistry 3Document7 pagesChemistry 3syed waseemNo ratings yet
- Chapter 3 Electro ChemistryDocument20 pagesChapter 3 Electro ChemistryKritika MishraNo ratings yet
- Electrochemistry Part 1Document35 pagesElectrochemistry Part 1ABHINAVNo ratings yet
- Electrolytic ProcessesDocument9 pagesElectrolytic ProcessesIshitha ChauhanNo ratings yet
- Corrosion MechanismsDocument64 pagesCorrosion MechanismsRahul PandeyNo ratings yet
- Iitm Diploma 1st Year Applied ChemistryDocument35 pagesIitm Diploma 1st Year Applied Chemistryneetugkp6388No ratings yet
- Unit 3 ElectrochemistryDocument14 pagesUnit 3 ElectrochemistrySuresh Dasaraddi100% (1)
- Electrochemistry Short NotesDocument5 pagesElectrochemistry Short Notestukutanigel423No ratings yet
- ElectrochemistryDocument2 pagesElectrochemistryria sNo ratings yet
- ElectroDocument13 pagesElectrodulalsushant3No ratings yet
- Concept of ElectrochemitryDocument15 pagesConcept of ElectrochemitryKritika SainiNo ratings yet
- Introduction To ElectrochemistryDocument40 pagesIntroduction To ElectrochemistryAngates1100% (2)
- ElectrochemistryDocument14 pagesElectrochemistryAMEEDA JABEENNo ratings yet
- Ec Notes - 11-7-18Document82 pagesEc Notes - 11-7-18Yasir AliNo ratings yet
- Electrochemical Cells: Electrode PotentialDocument20 pagesElectrochemical Cells: Electrode PotentialMunazNo ratings yet
- NEET Electrochemistry FinalDocument204 pagesNEET Electrochemistry Finaljaisree291006No ratings yet
- Chapter 04: Electrochemistry: Sheet-1Document35 pagesChapter 04: Electrochemistry: Sheet-1imtial kashemNo ratings yet
- 1219 159 578 Module 1Document86 pages1219 159 578 Module 1Mercy SajiNo ratings yet
- 2nd PhysicalDocument7 pages2nd Physicalsmarialatif11No ratings yet
- Section-A (Electro Chemistry) : A Brief Review of The Basic ConceptsDocument33 pagesSection-A (Electro Chemistry) : A Brief Review of The Basic ConceptsKanchanNo ratings yet
- Corrosion 4Document34 pagesCorrosion 4Faria Sultana MimiNo ratings yet
- Module 2 Notes Chem Mescenotes - inDocument31 pagesModule 2 Notes Chem Mescenotes - inHafizNo ratings yet
- A 197 - A 197M - 00 (2015)Document4 pagesA 197 - A 197M - 00 (2015)phaindikaNo ratings yet
- PHOTOVOLTAICSDocument27 pagesPHOTOVOLTAICSbrian otienoNo ratings yet
- CHT307 - Ktu QbankDocument3 pagesCHT307 - Ktu QbankYuxin CasioNo ratings yet
- Materials: Ffect of Different Compatibilizers On The Properties /poly (Butylene Adipate-CoDocument16 pagesMaterials: Ffect of Different Compatibilizers On The Properties /poly (Butylene Adipate-CoSiddharthBhasneyNo ratings yet
- 3G3mx2-v2 Ds e 1 1 csm1126864Document54 pages3G3mx2-v2 Ds e 1 1 csm1126864Pertti HänninenNo ratings yet
- Study of An Aquifer in A Semi-Arid Area Using MRS, FDEM, TDEM and ERT Methods (Youssoufia and Khouribga, Morocco)Document4 pagesStudy of An Aquifer in A Semi-Arid Area Using MRS, FDEM, TDEM and ERT Methods (Youssoufia and Khouribga, Morocco)tikroumineNo ratings yet
- Flow ChartDocument2 pagesFlow ChartJames PerriamNo ratings yet
- Module 2 Laplace TransformsDocument36 pagesModule 2 Laplace TransformsErnie Mark Patosa MaratasNo ratings yet
- Linear Motion Power Transmission Design Guide 02Document274 pagesLinear Motion Power Transmission Design Guide 02Ravindra KirangeNo ratings yet
- Schedule - JEE Advanced Crash Course 2024Document2 pagesSchedule - JEE Advanced Crash Course 2024saurav.saha1984No ratings yet
- IISER Syllabus: MathematicsDocument3 pagesIISER Syllabus: MathematicsIIT aspNo ratings yet
- Material List For PLC ProjectsDocument1 pageMaterial List For PLC ProjectsEngr Muhib KhÅñNo ratings yet
- Me6411 Manufacturing Technology-II Lab ManualDocument35 pagesMe6411 Manufacturing Technology-II Lab ManualdibyenindusNo ratings yet
- CM-PFS: Three-Phase Monitoring RelaysDocument10 pagesCM-PFS: Three-Phase Monitoring RelaysfirozNo ratings yet
- (NS) XII EM One Word Vol - IDocument12 pages(NS) XII EM One Word Vol - IAnishaNo ratings yet
- Is It Simple To Explain Simple Experiments The Heavy Newspaper' Stick BreakDocument6 pagesIs It Simple To Explain Simple Experiments The Heavy Newspaper' Stick Break이온유No ratings yet
- Anaytic Questioner - FinalDocument12 pagesAnaytic Questioner - FinalEdwin LoquinaNo ratings yet
- O Level Physics Text BookDocument111 pagesO Level Physics Text BookMatabaro BenjaminNo ratings yet
- Design of Helical SpringDocument11 pagesDesign of Helical SpringNarender Kumar100% (1)
- Lesson 10 - Haloalkanes & HaloarenesDocument170 pagesLesson 10 - Haloalkanes & HaloarenesAwez FahadNo ratings yet
- Module 4 Work Energy PowerDocument7 pagesModule 4 Work Energy PowerGreen BrainNo ratings yet
- Silicon Institute of Technology Department of Electrical & Electronics Engineering Quiz Test Electrical Machine-II Lab 5 Sem EEE-B, Gr.-I & IIDocument3 pagesSilicon Institute of Technology Department of Electrical & Electronics Engineering Quiz Test Electrical Machine-II Lab 5 Sem EEE-B, Gr.-I & IIT. VARMANo ratings yet
- EE6512-Electrical Machines LaboratoryDocument82 pagesEE6512-Electrical Machines LaboratoryGopinath B L NaiduNo ratings yet
- Write Answers To All NCERT Intext Solved & Unsolved Problems. 2. Write Answers To All NCERT Questions in ExercisesDocument2 pagesWrite Answers To All NCERT Intext Solved & Unsolved Problems. 2. Write Answers To All NCERT Questions in ExercisesJagriti DaryaniNo ratings yet
- Thermal ComfortDocument4 pagesThermal ComfortVidya HittiNo ratings yet
- Lecture - 8: Fluid Motion: Streamlines EtcDocument13 pagesLecture - 8: Fluid Motion: Streamlines Etcrohit singhNo ratings yet
- CSE 28363 - Structural Concrete Design: Lecture 10: Shear and ServiceabilityDocument44 pagesCSE 28363 - Structural Concrete Design: Lecture 10: Shear and ServiceabilityBitch CarrieNo ratings yet
- Temp4 Design ProceduresDocument23 pagesTemp4 Design ProceduresAhmed AdelNo ratings yet