Professional Documents
Culture Documents
Alanin
Alanin
Uploaded by
Hamed altaj0 ratings0% found this document useful (0 votes)
8 views7 pagesThis document summarizes the analysis of an unknown compound with the molecular formula C3H7NO2. The IR spectrum showed peaks indicating the presence of hydroxyl and carbonyl groups, suggesting a carboxylic acid. NMR analysis found two aliphatic carbon environments and one carbonyl carbon. Mass spectrometry revealed fragment patterns matching a molecular weight of 89, consistent with propanoic acid. Therefore, the compound was determined to be propanoic acid.
Original Description:
Spectroscopic analysis of alanin
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document summarizes the analysis of an unknown compound with the molecular formula C3H7NO2. The IR spectrum showed peaks indicating the presence of hydroxyl and carbonyl groups, suggesting a carboxylic acid. NMR analysis found two aliphatic carbon environments and one carbonyl carbon. Mass spectrometry revealed fragment patterns matching a molecular weight of 89, consistent with propanoic acid. Therefore, the compound was determined to be propanoic acid.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
8 views7 pagesAlanin
Alanin
Uploaded by
Hamed altajThis document summarizes the analysis of an unknown compound with the molecular formula C3H7NO2. The IR spectrum showed peaks indicating the presence of hydroxyl and carbonyl groups, suggesting a carboxylic acid. NMR analysis found two aliphatic carbon environments and one carbonyl carbon. Mass spectrometry revealed fragment patterns matching a molecular weight of 89, consistent with propanoic acid. Therefore, the compound was determined to be propanoic acid.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 7
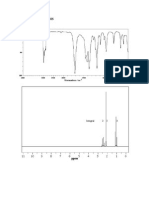
Problem 20:
IR:
From the IR there is absorption of broad peak between 3300-2500
indicates the presence of stretching of hydroxyl group (OH) and there
is absorption at 1620 cm which indicates the presence of stretching
carbonyl group.
Presence of carbonyl group and OH mean presence of carboxylic
acid.
Determination the IHD for unknown compound
C3H7NO2
CnH2n+2
×C3H8, 8-(7-1) =8-6=2,2\2=1→IHD→3+2=8 2=1
.One double bond of carboxylic acid
From C13 NMR we have 2 carbons
environments,2 aliphatic carbons between
.10ppm and 60ppm
From DEPT the aliphatic carbons maybe CH or(
)CH3 due to the peaks are upwards
and there is one carbonyl carbon between 170-
180ppm,there is no symetric carbons.
:From H NMR spectrum
there are 3 environments,doublet peak between 1.6-1.4ppm
indicates the presence of CH nearby this proton.Multiplet
peak between 3.9-3.7ppm indicates the presence of CH3
nearby this proton.Single peak 5-4ppm symbolizes for
.presence of NH2 and OH
:From Mass Spectrum
The fragmentation pattern show the molecular
formula is 89
the M.wt of CH3 15=89-74
.the M.wt of COOH 45=89-44
From the previous indexes we could conclude that
.the chemical structure of this compound
ALALIN
DONE BY:
Thekra Tateq
Asma`a Almahfadi
You might also like
- Spectra ProblemsDocument111 pagesSpectra ProblemsChandra Reddy100% (1)
- Spectroscopic Solutions of StructureDocument21 pagesSpectroscopic Solutions of StructureKassimNo ratings yet
- IR&NMR ProblemsDocument43 pagesIR&NMR ProblemsAndrew Ronaldi Tandio100% (2)
- Linkage 2 Lab ReportDocument25 pagesLinkage 2 Lab Reportapi-25176084883% (6)
- Spectroscopy Combined ProblemsDocument18 pagesSpectroscopy Combined ProblemsParyul ChaudhariNo ratings yet
- Notes 14C CNMRDocument5 pagesNotes 14C CNMRTux BsdNo ratings yet
- Solve The Following Spectral Problems As Far As Possible Give Possible Justifications. Also Predict The Fragmentation PatternDocument8 pagesSolve The Following Spectral Problems As Far As Possible Give Possible Justifications. Also Predict The Fragmentation Patternramesh pokhrelNo ratings yet
- Unit 14 PDFDocument20 pagesUnit 14 PDFAnonymous Y75qPkj2jNo ratings yet
- Hướng Dẫn Lẫm Bẫi Tẫp Kết Hớp Phổ Hổng Ngổẫi Vẫ Khổi PhổDocument19 pagesHướng Dẫn Lẫm Bẫi Tẫp Kết Hớp Phổ Hổng Ngổẫi Vẫ Khổi PhổAn Lê TrườngNo ratings yet
- C-13 NMR and DEPTDocument41 pagesC-13 NMR and DEPTV G Viju Kumar100% (1)
- L3 NMR 1Document13 pagesL3 NMR 1Cheng FuNo ratings yet
- OrganicChemistry10e SSM Ch12Document18 pagesOrganicChemistry10e SSM Ch12UNBROKEN BONDNo ratings yet
- SCHB032 - Memo - Test 1 2022Document5 pagesSCHB032 - Memo - Test 1 2022emjayNo ratings yet
- 39 SpectrosDocument9 pages39 SpectrosDinel JosephNo ratings yet
- Spectral For PrintingDocument15 pagesSpectral For PrintingChandra ReddyNo ratings yet
- Assignment 2 - Pool of Questions - SolutionDocument11 pagesAssignment 2 - Pool of Questions - SolutionNishant KhandelwalNo ratings yet
- 13C NMRDocument28 pages13C NMRArjun MaharajNo ratings yet
- Kalbus 1991Document2 pagesKalbus 1991zara.khan0013No ratings yet
- IR - HNMR ProblemsDocument33 pagesIR - HNMR Problemsbsakaly112100% (1)
- 02 SpectroscopystratDocument3 pages02 SpectroscopystratDhiraj PatilNo ratings yet
- NMR Multiple Choice Questions PDFDocument71 pagesNMR Multiple Choice Questions PDFDeepak SinghNo ratings yet
- Topic 20 Answers To ExercisesDocument4 pagesTopic 20 Answers To ExercisesSiti NuraqidahNo ratings yet
- 13C NMRDocument40 pages13C NMRKrishna BurakaNo ratings yet
- Key HW 3 Part II SpecDocument16 pagesKey HW 3 Part II SpecTha KantanaNo ratings yet
- NMR Solving StrategyDocument2 pagesNMR Solving Strategysorrow Lemon100% (1)
- Cu ComplexesDocument8 pagesCu ComplexesvicianmNo ratings yet
- 2013 Alkane Tutorial (Solutions)Document7 pages2013 Alkane Tutorial (Solutions)Pinzhen ChenNo ratings yet
- Topic 10 Alkane TutorialDocument6 pagesTopic 10 Alkane TutorialTimNo ratings yet
- 13-C NMR-09Document27 pages13-C NMR-09M Nur M. MahmudNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- NMR - Multiple Choice QuestionsDocument71 pagesNMR - Multiple Choice QuestionsOmSilence265171% (31)
- Clayden 2e - End of Chapter Questions - Ch3Document4 pagesClayden 2e - End of Chapter Questions - Ch3Nikola NinkovNo ratings yet
- Guía de Estudio EspectrosDocument5 pagesGuía de Estudio EspectrosCésar CidNo ratings yet
- Engr M Ali BhuttaDocument13 pagesEngr M Ali Bhuttahashrox1No ratings yet
- CHM580-Tutorial IR Dan RamanDocument6 pagesCHM580-Tutorial IR Dan RamanSuhailyShukri100% (1)
- 13C NMR Spectroscopy Power Point PresentationDocument34 pages13C NMR Spectroscopy Power Point PresentationSagarChedeNo ratings yet
- Practice Questions For Test 2, Spring 2015Document10 pagesPractice Questions For Test 2, Spring 2015Arianne Foster100% (1)
- L2 UV-VisDocument17 pagesL2 UV-VisCheng FuNo ratings yet
- Halogenacion de AlcanosDocument34 pagesHalogenacion de AlcanosAlejandra EcheverriNo ratings yet
- Zuo 2016Document10 pagesZuo 2016Phạm NgânNo ratings yet
- Organic Chemistry 2: Number of C ×Document5 pagesOrganic Chemistry 2: Number of C ×Trung VõNo ratings yet
- Spectroscopy ManualDocument21 pagesSpectroscopy Manualanthor100% (2)
- NSKH MN Presentation1 240 Chem 1 0Document37 pagesNSKH MN Presentation1 240 Chem 1 0batazaiNo ratings yet
- Solved Examples: CH CH + CL CH CH CLDocument10 pagesSolved Examples: CH CH + CL CH CH CLHarsh TyagiNo ratings yet
- SrO Short CommunicationDocument5 pagesSrO Short CommunicationGeorgina De MiccoNo ratings yet
- Assignment 1 CHM557Document15 pagesAssignment 1 CHM557Jibb BossNo ratings yet
- BBPC 19820860703Document11 pagesBBPC 19820860703Bernardo MacaraNo ratings yet
- The Growth Fission Gas Bubbles in Irradiated Uranium DioxideDocument16 pagesThe Growth Fission Gas Bubbles in Irradiated Uranium DioxideGwanyun JeongNo ratings yet
- Topic 15 Organic Chemistry: Carbonyls, Carboxylic Acids and ChiralityDocument9 pagesTopic 15 Organic Chemistry: Carbonyls, Carboxylic Acids and ChiralitysalmaNo ratings yet
- 13C NMR Spectroscopy Power Point PresentationDocument34 pages13C NMR Spectroscopy Power Point PresentationOti DeeaNo ratings yet
- General Organic Chemistry Theory 1Document28 pagesGeneral Organic Chemistry Theory 1OasisEducation OesNo ratings yet
- 1991-Determination of Degree of Deacetylation of Chitosan by 1H NMR SpectrosDocument8 pages1991-Determination of Degree of Deacetylation of Chitosan by 1H NMR SpectrosBashir BetarNo ratings yet
- Ijcb 44B (6) 1239-1242Document4 pagesIjcb 44B (6) 1239-1242Sarathkumar PathivadaNo ratings yet
- A16 - Spectrocscopic IdentificationDocument2 pagesA16 - Spectrocscopic IdentificationAlyasin FrougaNo ratings yet
- Organic Chemistry 2Document5 pagesOrganic Chemistry 2Trung VõNo ratings yet
- 3 - Carbon-13-NMRDocument21 pages3 - Carbon-13-NMRahmed mohamedNo ratings yet
- A Level Chemistry Paper 1 Set 2 Marking GuideDocument7 pagesA Level Chemistry Paper 1 Set 2 Marking Guidessentume peterNo ratings yet
- bài tập phổ nrmDocument102 pagesbài tập phổ nrmĐức NamNo ratings yet
- NMR 13C 13 QuesDocument52 pagesNMR 13C 13 QuesgussalimNo ratings yet
- Electrochemical Processes in Biological SystemsFrom EverandElectrochemical Processes in Biological SystemsAndrzej LewenstamNo ratings yet