Professional Documents
Culture Documents
Ozone Hole
Ozone Hole
Uploaded by
akash rathi0 ratings0% found this document useful (0 votes)
4 views9 pagesOriginal Title
ozone hole
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
4 views9 pagesOzone Hole
Ozone Hole
Uploaded by
akash rathiCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 9
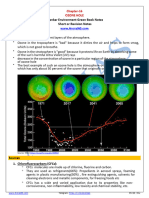
OZONE HOLE
• It is found in two different layers of the
atmosphere.
• Ozone in the troposphere is "bad"
because it dirties the air and helps to
form smog, which is not good to breathe.
• Ozone in the stratosphere is "good"
because it protects life on Earth by
absorbing some of the sun's harmful
Ultra Violet (UV) rays
• decrease in the concentration of ozone in
a particular region of the atmosphere -
'ozone hole'
• The best example of such an ozone hole
is theatmosphere over the Antarctic
which has only about 50 percent of the
ozone that originally occurred there.
Sources
• Chlorofluorocarbons (CFCs):
• CFCs molecules are made up of chlorine, fluorine and
carbon.
• They are used as refrigerants(66%) ; propellants in aerosol
sprays, foaming agents in plastic manufacturing(30%) , fire
extinguishing agents, solvents for cleaning electronic and
metallic components, for freezing foods etc
• CFCs has a wide and varied application due to its properties
like non-corrosiveness, non-inflammability, low toxicity and
chemical stability, etc.
• the residence time of CFCs in the atmosphere estimated to
be between 40 and 150 years
• The chemical reaction
• CFCs + UV radiation = freeing chlorine atoms.
• A free chlorine atom + ozone molecule to = chlorine
monoxide (00).
• chlorine monoxide + atom of oxygen.= (02) and reformation
of the free chlorine atom (CI).
• The element that destroys 03 (i.e chlorine) is being reformed
at the end of cycle.
• A single chlorine atom destroy thousands of ozone molecules
before encountering reactive nitrogen or hydrogen
• Nitrogen Oxides:
• The chemical reaction- Nitric oxide (NO)
catalytically destroys ozone.
• Nitric oxide + ozone =Nitrogen dioxide +
o2
• Nitrogen dioxide + monoxide =Nitric oxide
+ Oxygen
• Bromine
• containing compounds called halons and
HBFCs, i.e. hydrobromo fluorocarbons
[both used in fire extinguishers and
methyl bromide (a widely used pesticide)].
• Each bromine atom destroys hundred
times of more ozone molecules than what
a chlorine atom does.
• Sulphuric acid particles:
• These particles free chlorine from
molecular reservoirs, and convert reactive
nitrogen into inert forms thus preventing,
the formation of chlorine reservoirs.
Role of polar stratospheric clouds in ozone depletion
• The ice particles of the cloud provided
substrates for chemical reactions which
freed chlorine from its reservoirs.
• the reaction between HC1 and C1ONO2 is
very slow, but this reaction occurs at a
faster rate in the presence of a suitable
substrate which is provided by the
stratospheric clouds at the poles.
• The PSCs not only activate chlorine, but
they also absorb reactive nitrogen.
• If nitrogen, - oxides were present they
would combine with chlorine monoxide to
form a reservoir of chlorine nitrate,
(ClONO2).
• Every spring, a hole as big as the USA
develops in the ozone layer over Antarctica,
in the South Pole.
• A smaller hole develops each year over the
Why is the ozone hole predominant at
the Antarctic?
• The Antarctic stratosphere is much
colder. The low temperature enables
the formation of Polar stratospheric
Clouds (PSCs), below 20 km
• The vortex is a ring of rapidly
circulating air that confines the ozone
depletion in the Antarctic region.
• The longetivity of the Antarctic vortex
is another factor, enhancing favorable
conditions for the depletion of ozone.
• The vortex remains, in fact,
throughout the polar winter, well into
midspring Whereas-the vortex in the
Arctic disintegrates by the time the
polar spring (March-April) arrives.
• The ozone measurement instruments and
techniques are varied. Some of them are the
Dobson spectrophotometer and the filter
ozonometer called M83, and total ozone mapping
pectrometer (TOMS) in the Nimbus-7 satellite.
• The Umheher technique- The most common
measure of total ozone abundance is the Dobson
unit (named afterthe pioneering atmospheric
physical Gordon Dobson) which is the thickness of
the ozone column (compressed at Standard
Temperature and Pressure (STP)) in milli-
centimeters.
You might also like
- Shankar Environment Green Book Notes Short or Revision NotesDocument3 pagesShankar Environment Green Book Notes Short or Revision NotessekkharNo ratings yet
- The Ozone Layer: Formation and DepletionDocument34 pagesThe Ozone Layer: Formation and DepletionAshutosh PandeyNo ratings yet
- The Ozone LayerDocument34 pagesThe Ozone LayerChiranjeet GhoshNo ratings yet
- Ozone LayerDocument21 pagesOzone LayerChandrima MannaNo ratings yet
- Ozone Layer DepletionDocument24 pagesOzone Layer DepletionJayan GjNo ratings yet
- Ozone Depletion - Chemistry ProjectDocument11 pagesOzone Depletion - Chemistry ProjectsujsamNo ratings yet
- Ozone Layer Depletion, Its Causes and Its EffectsDocument24 pagesOzone Layer Depletion, Its Causes and Its EffectsPrince SultanNo ratings yet
- Ozone DepletionDocument22 pagesOzone Depletionhcl1239704No ratings yet
- Ozone Depletion: From Wikipedia, The Free EncyclopediaDocument23 pagesOzone Depletion: From Wikipedia, The Free EncyclopediaKrishnakant BajpaiNo ratings yet
- Ozone DepletionDocument25 pagesOzone DepletionEl AusNo ratings yet
- Lec-4 (Environmental Issues)Document40 pagesLec-4 (Environmental Issues)Arsalan AliNo ratings yet
- Ozone Depletion: Navigation SearchDocument22 pagesOzone Depletion: Navigation SearchNupur BhadraNo ratings yet
- Ozone Layer DepletionDocument12 pagesOzone Layer Depletionnandkishore_singhNo ratings yet
- Global Environmental ConcernsDocument20 pagesGlobal Environmental ConcernsNandini KotharkarNo ratings yet
- Understanding & Tracking Antarctica's Ozone HoleDocument24 pagesUnderstanding & Tracking Antarctica's Ozone HoleExamNo ratings yet
- FSN 1500 Week 12: Stratospheric Ozone Depletion, Ground-Level Ozone and The Electromagnetic SpectrumDocument90 pagesFSN 1500 Week 12: Stratospheric Ozone Depletion, Ground-Level Ozone and The Electromagnetic SpectrumJitendra YadavNo ratings yet
- What Is The Ozone Layer?Document4 pagesWhat Is The Ozone Layer?yskstogtyNo ratings yet
- The Ozone Layer: Formation and DepletionDocument34 pagesThe Ozone Layer: Formation and Depletionyksingla37No ratings yet
- The Ozone LayerDocument34 pagesThe Ozone LayerHuma HaiderNo ratings yet
- Lec-7 Atmos Chem of OzoneDocument46 pagesLec-7 Atmos Chem of OzoneWewewe WeweweNo ratings yet
- The Ozone Layer (The Good Ozone) : AuroraDocument16 pagesThe Ozone Layer (The Good Ozone) : AurorapgbernsteinNo ratings yet
- Ozone DepletionDocument17 pagesOzone DepletionNaseema MalikNo ratings yet
- Ozone Layer Depletion, Its Causes and Its EffectsDocument24 pagesOzone Layer Depletion, Its Causes and Its EffectsDarya MemonNo ratings yet
- In Partial Fulfillment in Science 2: Submitted ToDocument21 pagesIn Partial Fulfillment in Science 2: Submitted ToTrialhyn YanoNo ratings yet
- The Ozone Layer: Formation and DepletionDocument34 pagesThe Ozone Layer: Formation and DepletionSwapnil DoshiNo ratings yet
- OzonDocument17 pagesOzonFaiqa NaazNo ratings yet
- We're Celebrating Wikipedia's 10th AnniversaryDocument7 pagesWe're Celebrating Wikipedia's 10th Anniversaryprad_jaiNo ratings yet
- Ozone DepletionDocument14 pagesOzone DepletionJoseph GratilNo ratings yet
- Lecture 2 - The Ozone Layer & Ozone Hole (Page 222 of First Text, But Mostly From Book "Earth Under Siege")Document25 pagesLecture 2 - The Ozone Layer & Ozone Hole (Page 222 of First Text, But Mostly From Book "Earth Under Siege")Salsabila Ainun NisaNo ratings yet
- Ozone DepletionDocument24 pagesOzone DepletionKyla de Silva100% (1)
- The Ozone Layer: Submitted BY Abhishek PrakashDocument9 pagesThe Ozone Layer: Submitted BY Abhishek PrakashAbhishek PrakashNo ratings yet
- Assignment of ENVIRONMENMTDocument16 pagesAssignment of ENVIRONMENMTRana HussnainNo ratings yet
- Ozone Layer - Significance, Formation and DepletionDocument16 pagesOzone Layer - Significance, Formation and Depletionsree TharunNo ratings yet
- Ozone DepletionDocument2 pagesOzone DepletionrenroseloraNo ratings yet
- Meteorologi ITB - Mata Kuliah Perubahan Iklim - 04 Ozon LayerDocument82 pagesMeteorologi ITB - Mata Kuliah Perubahan Iklim - 04 Ozon LayerDinda SNo ratings yet
- Ozone Layer ProjectDocument20 pagesOzone Layer ProjectAMIN BUHARI ABDUL KHADER100% (5)
- Environmental Impact and Human Health Impact of Refrigeration & Air-ConditioningDocument18 pagesEnvironmental Impact and Human Health Impact of Refrigeration & Air-ConditioningVasti Diaz AguilarNo ratings yet
- Environmental Science and Engineering enDocument50 pagesEnvironmental Science and Engineering enWilliam Tac anNo ratings yet
- Eman Salah Nader Sameh Merna Rafaat Heba Ali Gihan Tawfik Sondos Amin Marim Mostafa Farah Sara Tarek 11 CDocument7 pagesEman Salah Nader Sameh Merna Rafaat Heba Ali Gihan Tawfik Sondos Amin Marim Mostafa Farah Sara Tarek 11 CEman MetwallyNo ratings yet
- 1 Environmental Chemistry The AtmosphereDocument33 pages1 Environmental Chemistry The AtmosphereHakdog playsNo ratings yet
- Stratospheric Ozone:: Production, Destruction, & TrendsDocument54 pagesStratospheric Ozone:: Production, Destruction, & TrendsChibo KunNo ratings yet
- The Ozone Layer: Formation and DepletionDocument34 pagesThe Ozone Layer: Formation and DepletionAdarsh LalitNo ratings yet
- OzoneLayer DepletionDocument44 pagesOzoneLayer Depletionaditya kumarNo ratings yet
- The Ozone LayerDocument8 pagesThe Ozone LayerAzam SidekNo ratings yet
- Stratospheric PhotolysisDocument7 pagesStratospheric PhotolysisKara BaigNo ratings yet
- Ozone Layer and Its ImportanceDocument5 pagesOzone Layer and Its ImportanceNaman VishwakarmaNo ratings yet
- Ozone Layer - Depletion and RecoveryDocument56 pagesOzone Layer - Depletion and RecoveryLiyaNo ratings yet
- Project On Ozone DepletionDocument18 pagesProject On Ozone DepletionakhileshNo ratings yet
- Earth Subsystems ATMOSPHEREDocument30 pagesEarth Subsystems ATMOSPHEREbae joohyunNo ratings yet
- 1 2 3 4 5 MergedDocument212 pages1 2 3 4 5 Mergedaravind100305No ratings yet
- CHM576 Lec1Document68 pagesCHM576 Lec1zatty kimNo ratings yet
- OzoneDocument4 pagesOzonejanson1523No ratings yet
- ch13 Sec2Document23 pagesch13 Sec2api-272720493No ratings yet
- Ozone LayerDocument2 pagesOzone LayerPatrick NaldaNo ratings yet
- Ozone Layer: Its Depletion and ImpactsDocument26 pagesOzone Layer: Its Depletion and ImpactsDishaNo ratings yet
- What Is The Ozone Layer and Where Is It Located?Document6 pagesWhat Is The Ozone Layer and Where Is It Located?JoseNo ratings yet
- All About The Ozone Layer : Effects on Human, Animal and Plant Health - Environment Books | Children's Environment BooksFrom EverandAll About The Ozone Layer : Effects on Human, Animal and Plant Health - Environment Books | Children's Environment BooksNo ratings yet
- Fun Facts about Oxygen : Chemistry for Kids The Element Series | Children's Chemistry BooksFrom EverandFun Facts about Oxygen : Chemistry for Kids The Element Series | Children's Chemistry BooksNo ratings yet
- Science For KidsDocument20 pagesScience For KidsKlemen Strušnik100% (1)
- Grade 07 Science 1st Term Test Paper 2018 English Medium Western ProvinceDocument6 pagesGrade 07 Science 1st Term Test Paper 2018 English Medium Western ProvinceFawzar Sabir75% (4)
- R744 Guide Parts 1 2 3Document182 pagesR744 Guide Parts 1 2 3alessandro silvaNo ratings yet
- HQ Rawang - Meeting - Materialsdesign - ChangesDocument3 pagesHQ Rawang - Meeting - Materialsdesign - ChangesAmirul ShamNo ratings yet
- Lab 7 Paper ChromatographyDocument7 pagesLab 7 Paper ChromatographyAhmadNo ratings yet
- Chapter 1 - Engineering OverviewDocument32 pagesChapter 1 - Engineering OverviewNor syaza syafmiza binti zulkifliNo ratings yet
- Glycerol Propylene. Reaction Scheme: EpichlorohydrinDocument3 pagesGlycerol Propylene. Reaction Scheme: EpichlorohydrinMUHAMMED BAQA ANWER100% (1)
- Fundamental PV Tech-Mod 02 Sunlight Solar Cell BasicsDocument53 pagesFundamental PV Tech-Mod 02 Sunlight Solar Cell BasicsSultan ZahidNo ratings yet
- Aluminum Sesquichlorohydrate SolutionDocument1 pageAluminum Sesquichlorohydrate SolutionK.m. Ehsan Morshed RanaNo ratings yet
- Beams With OpeningsDocument7 pagesBeams With OpeningshemanthyadavNo ratings yet
- Chemical BOL DETAILSDocument3 pagesChemical BOL DETAILSJonny DenNo ratings yet
- PEARSON Chemistry Chapter 9 Flashcards - QuizletDocument4 pagesPEARSON Chemistry Chapter 9 Flashcards - Quizletأستغفرالله واتوب اليهNo ratings yet
- Laboratory Test EquipmentDocument3 pagesLaboratory Test EquipmentRaqibul HasanNo ratings yet
- Fracture Mechanics - MUHAMMADABDULLAH ATIFDocument10 pagesFracture Mechanics - MUHAMMADABDULLAH ATIFSyed Hassan.911No ratings yet
- A Study of Oxygen Separation From Air by PSA ProcessDocument12 pagesA Study of Oxygen Separation From Air by PSA ProcesspolysourceNo ratings yet
- Membrane Cleaning - Cost Effective Solution: Kimberlite Chemicals India Private LimitedDocument65 pagesMembrane Cleaning - Cost Effective Solution: Kimberlite Chemicals India Private LimitedAshish RathoreNo ratings yet
- Ss Bag Filter HousingDocument5 pagesSs Bag Filter HousingisepcontrolNo ratings yet
- Elbows - DIN 2605: VL Code 711Document3 pagesElbows - DIN 2605: VL Code 711ramonaghergheNo ratings yet
- Danieli Corus SublanceDocument4 pagesDanieli Corus SublanceBinay Kumar ChoudharyNo ratings yet
- Chapter 3Document19 pagesChapter 3viaalcantsNo ratings yet
- American Galvanizers Association-Hot-Di P Galvanizing vs. Paint in Life-Cycle Assessment PDFDocument4 pagesAmerican Galvanizers Association-Hot-Di P Galvanizing vs. Paint in Life-Cycle Assessment PDFdgkmurtiNo ratings yet
- ASTM E291-01 Standard Test Methods For Chemical Analysis of Caustic Soda and Caustic PotashDocument14 pagesASTM E291-01 Standard Test Methods For Chemical Analysis of Caustic Soda and Caustic PotashWijianto WijiantoNo ratings yet
- TranspirationDocument22 pagesTranspirationyr44grf94kNo ratings yet
- Carbohydrate MetabolismDocument8 pagesCarbohydrate MetabolismChhan Kumar kalitaNo ratings yet
- Coal Fired Boiler - PrincipalsDocument145 pagesCoal Fired Boiler - PrincipalsArfan AnwarNo ratings yet
- Ion Gauge TheoryDocument14 pagesIon Gauge TheoryDan Ste MarieNo ratings yet
- National Level Science Talent Search Examination: Test Assess AchieveDocument6 pagesNational Level Science Talent Search Examination: Test Assess AchievevirajNo ratings yet
- Kit Insert Magnesium (Calmagite)Document2 pagesKit Insert Magnesium (Calmagite)rai citaNo ratings yet
- M2-Combustion ThermodynamicsDocument3 pagesM2-Combustion ThermodynamicsKrishna KumarNo ratings yet
- Material Safety Data Sheet: Section I - IdentificationDocument2 pagesMaterial Safety Data Sheet: Section I - IdentificationMohamed AdelNo ratings yet