Professional Documents
Culture Documents
Nuclear Chemistry - 2
Nuclear Chemistry - 2
Uploaded by
Simon Lucian0 ratings0% found this document useful (0 votes)
7 views21 pagesOriginal Title
Nuclear Chemistry -2
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
0 ratings0% found this document useful (0 votes)
7 views21 pagesNuclear Chemistry - 2
Nuclear Chemistry - 2
Uploaded by
Simon LucianCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
Download as pptx, pdf, or txt
You are on page 1of 21
NUCLEAR CHEMISTRY
The atom: History
• Democritus :400BC
• Atom=atomos (not to be cut) by a Greek
philosopher who began the search for a
description of matter
The atomic model has
changed throughout the
centuries, starting in 400 BC,
when it looked like a billiard
ball . The theory was ignored
for more than 2000 years
Aristotle and Plato
• favored the earth, fire, air
and water approach to the
nature of matter. Their ideas
held sway because of their
eminence as philosophers.
Originally proposed by
Empedocles in 450 BC. The
atomos idea was buried for
approximately 2000 years.
More models….
• Dalton
• Rutherford
• Bohr
• Modern (summary)
• Wave model
• THE BOHR MODEL
In this model, the
nucleus is orbited by
electrons, which are
in different
energy levels.
Daltons atomic theory
• Postulates
Summary
Indivisible Electron Nucleus Orbit Electron
Cloud
Greek X
Dalton X
Thomson X
Rutherford X X
Bohr X X X
Wave X X X
THE ATOMIC STRUCTURE
• The atom
• Subatomic particles
• Electron: discovery and properties
• The nucleus: ,,
• Proton: ,,
• Neutron: ,,
Some definitions
• Atomic number
• Mass number
• Chemical element
• Nuclide
• Isotopes
• Isotones
• Isobars
• Isodiaphers
Some definitions..
• System
• Surroundings
• Boundary
• Open
• Closed
• Isolated
Law of conservation of Mass – Energy
• The law of conservation of mass or the principle of
mass/matter conservation, states that the mass of
an isolated system (closed to all matter and energy)
will remain constant over time (*give examples)
• The law of conservation of energy (1st law of
thermodynamics): the energy of an isolated system
remains constant.
• The law of conservation of mass-energy: the total
amount of mass and energy in the universe is
constant. E α m, E=mc2
Mass defect
• Represents the amount of matter that would be
converted into energy and released if the
nucleus were formed from separate neutrons
and protons.
• The difference between the mass of a nucleus
and the sum of the masses of the nucleons of
which it is composed is called the mass defect.
• *Use Cu-63 to explain mass defect and do some
calculations
Binding Energy
• Nuclear binding energy: is the energy
required to break down a nucleus into its
component nucleons or energy involved in
the combination of nucleons to form a
nucleus. It is in essence the quantitative
measure of nuclear stability .
• Binding energy is based on Einstein's
equation E=mc2
Binding Energy..
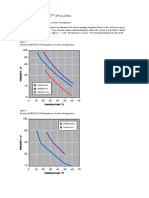
The Binding Energy Per Nucleon as a Function of Mass Number
Nuclear stability.
• A stable nuclide
• Determinants of nuclear stability:
• (i) Nuclear forces (ii) odd-even rule (iii)
magic numbers (iv) n/p on N/Z ratio
Nuclear stability..
• i. Nuclear forces
• Neutron-to-neutron
• Proton-to-proton
• Proton-neutron
• Electromagnetic forces
• Weak forces
Nuclear stability...
• ii. Nuclides with even numbers of protons and
neutrons are more stable.
• In general, nuclear stability is greater for nuclides
containing even numbers of protons and neutrons
or both.
•
Nuclear stability….
• iii. Specific numbers of protons or neutrons
(magic numbers) such as 2, 8, 20, 28, 50,
82, and 126 produce stable nuclides
• For example: 42He, 168O, and 4020Ca. One
stable isotope of lead, 208 82 Pb, has 82
protons and 126 neutrons.
Nuclear stability…..
• All nuclides with 84 or more
protons are unstable with
respect to radioactive decay.
• Light nuclides are stable when
neutron/proton = 1.
• For heavier elements the
neutron /proton ratio required
for stability is greater than 1
and increases with Z.
End
You might also like
- As 1726-2017Document81 pagesAs 1726-2017Darren Newell25% (4)
- Lab 8Document7 pagesLab 8Brandon Sookdeo100% (4)
- Isolation of DNA Extraction From Plant TissueDocument6 pagesIsolation of DNA Extraction From Plant Tissueanura7No ratings yet
- Radiation Physics LectureDocument272 pagesRadiation Physics LectureMark M. AlipioNo ratings yet
- First Year Chemistry 20-02Document100 pagesFirst Year Chemistry 20-02Ahmed Hassan Mina HamadNo ratings yet
- Lecture 2. Atomic Structure and Interatomic BondingDocument69 pagesLecture 2. Atomic Structure and Interatomic BondinggiftstephensmwaleNo ratings yet
- Atomic Structure: Vinay Desai M.SC Radiation Physics Kidwai Memorial Institute of OncologyDocument28 pagesAtomic Structure: Vinay Desai M.SC Radiation Physics Kidwai Memorial Institute of OncologyJerry De Leon LptNo ratings yet
- Atomic Structure: Vinay Desai M.SC Radiation Physics Kidwai Memorial Institute of OncologyDocument28 pagesAtomic Structure: Vinay Desai M.SC Radiation Physics Kidwai Memorial Institute of OncologyJerry De Leon LptNo ratings yet
- S1 2022 P Fisika - Inti I - Struktur Inti AtomDocument64 pagesS1 2022 P Fisika - Inti I - Struktur Inti AtomMuhammad Agustian AdhityaNo ratings yet
- Structure of Atom: Made By:dr. Isha Jaiswal Moderator: Dr. S.P.MishraDocument37 pagesStructure of Atom: Made By:dr. Isha Jaiswal Moderator: Dr. S.P.MishraRafael OrtegaNo ratings yet
- PM1 and Prepharm 2022 Lecture SlidesDocument225 pagesPM1 and Prepharm 2022 Lecture SlidesMary KallonNo ratings yet
- NuclearDocument117 pagesNuclearsarfaraz khanNo ratings yet
- EE2317-Course-Atomic Structures and Interatomic BondingDocument94 pagesEE2317-Course-Atomic Structures and Interatomic BondingJOSEPH BENEDICT PRIMNo ratings yet
- ANFT1101Document60 pagesANFT1101ggdd4328No ratings yet
- The Atom For AnatomyDocument106 pagesThe Atom For AnatomyAlexandra B. FloresNo ratings yet
- 01-Atomic N Nuclear StructureDocument23 pages01-Atomic N Nuclear StructureEngr Umair AzizNo ratings yet
- Chapter 4 - Structure of The AtomDocument59 pagesChapter 4 - Structure of The Atomsivenday21No ratings yet
- Atomic Nucleus and RadioactivityDocument59 pagesAtomic Nucleus and Radioactivitymladen lakic100% (2)
- Chemistry: Atoms, Molecules, & Chemical BondingDocument149 pagesChemistry: Atoms, Molecules, & Chemical BondingnadnotmeNo ratings yet
- Electrical Engineering Science 1 - 230419 - 164951Document109 pagesElectrical Engineering Science 1 - 230419 - 164951Akogun ElizabethNo ratings yet
- Introduction To Organic Chemistry: Lesson: 1Document44 pagesIntroduction To Organic Chemistry: Lesson: 1Nikol BaltazarNo ratings yet
- Chapter-4: Structure of An AtomDocument20 pagesChapter-4: Structure of An AtomTanisha JainNo ratings yet
- Structure of An Atom: Background KnowledgeDocument107 pagesStructure of An Atom: Background KnowledgeTuqir BaabayNo ratings yet
- Radiologic Physics I: Amada, RRTDocument81 pagesRadiologic Physics I: Amada, RRTHelen RubianNo ratings yet
- Chemistry 101-WI50 Lecture 2Document31 pagesChemistry 101-WI50 Lecture 2JohnfedoNo ratings yet
- ACIS Atomic StructureDocument31 pagesACIS Atomic StructureJeenal AgrawalNo ratings yet
- 08 The Atom SlidesDocument26 pages08 The Atom Slidesjackoread28No ratings yet
- Atoms MoleculesDocument78 pagesAtoms MoleculesShanenely minNo ratings yet
- CERAMICS - II-week IDocument123 pagesCERAMICS - II-week IsniremapakNo ratings yet
- REVIEWER OF GEN CHEM ^0 RWS 2ND SUMMATIVE TESTDocument8 pagesREVIEWER OF GEN CHEM ^0 RWS 2ND SUMMATIVE TEST11boyle.locsinjanilleNo ratings yet
- 6B Atomic StructureDocument137 pages6B Atomic StructureOrane CassanovaNo ratings yet
- Chapter 3 AtomsDocument98 pagesChapter 3 AtomsAriana cruzNo ratings yet
- Topic 1 Basic Physics For Nuclear Medicine PDFDocument66 pagesTopic 1 Basic Physics For Nuclear Medicine PDFJeeShaoMinNo ratings yet
- Atomic StructureDocument6 pagesAtomic StructureAnabella MaldonadoNo ratings yet
- Chapter 4 - Structure of The AtomDocument59 pagesChapter 4 - Structure of The AtomIsaac LibuNo ratings yet
- Atomic Structure Atoms and IonsDocument61 pagesAtomic Structure Atoms and IonsRishi Kant SharmaNo ratings yet
- General Chemistry Lecture-Chapter 1Document78 pagesGeneral Chemistry Lecture-Chapter 1Bảo Long Trần LêNo ratings yet
- CSEC Physics - The AtomDocument5 pagesCSEC Physics - The AtomCornflakes ToastedNo ratings yet
- Atomic StructureDocument33 pagesAtomic Structureshikshyapokhrel3003No ratings yet
- Chapter - 4 "Structure of Atom" Concept Details Key ConceptsDocument11 pagesChapter - 4 "Structure of Atom" Concept Details Key ConceptsKishlay AnandNo ratings yet
- Atomic Structure PDFDocument59 pagesAtomic Structure PDFNashraat BukhoryNo ratings yet
- Lecture 3Document21 pagesLecture 3ethio universeNo ratings yet
- Chapter 2-2Document31 pagesChapter 2-2fatemaNo ratings yet
- Chapter 1Document150 pagesChapter 1FaaiqahNasaryNo ratings yet
- Introduction To Nuclear EngineeringDocument58 pagesIntroduction To Nuclear EngineeringTanzim Rafat AyonNo ratings yet
- 1.1 The Nature of Atoms Jan 2019Document37 pages1.1 The Nature of Atoms Jan 2019Dima SabeehNo ratings yet
- CH205Atomic Structure StudentDocument63 pagesCH205Atomic Structure Studentpravishek maniNo ratings yet
- 6 Atoms, Molecules Merged Lecture Note1-4Document98 pages6 Atoms, Molecules Merged Lecture Note1-4Stanley OgiliNo ratings yet
- Atomic Structure: Name: Mr. Burnett Date: 03/05/2021 Class: 6A PhysicsDocument50 pagesAtomic Structure: Name: Mr. Burnett Date: 03/05/2021 Class: 6A PhysicsACSVNo ratings yet
- Unit I Nuclear Structure & RadioactivityDocument66 pagesUnit I Nuclear Structure & RadioactivityAnupriya AnuNo ratings yet
- Chapter-1 Atomic Structures and Theory-FinalDocument138 pagesChapter-1 Atomic Structures and Theory-FinalJATIN DALMIANo ratings yet
- General Inorganic Chemistry Presentation For BSU Compre Handout 2Document118 pagesGeneral Inorganic Chemistry Presentation For BSU Compre Handout 2Ahe BeongNo ratings yet
- Atoms and The Atomic TheoryDocument47 pagesAtoms and The Atomic TheoryEdgar PeninsulaNo ratings yet
- Chapter 4 (Atoms and Atomic Theory)Document47 pagesChapter 4 (Atoms and Atomic Theory)Raynan TabaldoNo ratings yet
- Chapter 4, Section 2Document20 pagesChapter 4, Section 2Abdullah AlthaniNo ratings yet
- Lec2 Atomic Bonding FinalDocument106 pagesLec2 Atomic Bonding FinalMuhammad LuqmanNo ratings yet
- AQA GCE Physics A Unit 1 - Revision NotesDocument18 pagesAQA GCE Physics A Unit 1 - Revision NotesJayprashant 'JP' JeyachandranNo ratings yet
- Particle Physics 221216 121646Document44 pagesParticle Physics 221216 121646Nur Ainna LiyanaNo ratings yet
- Wala ToDocument168 pagesWala ToBlaize Aurellius B. CordeneteNo ratings yet
- Nuclear Chemistry 20-10-2020Document16 pagesNuclear Chemistry 20-10-2020Manohar MaripeNo ratings yet
- Basic Radiation Concepts Week 8Document36 pagesBasic Radiation Concepts Week 8melfordbatucanNo ratings yet
- Carbon Compounds and Chemical BondsDocument50 pagesCarbon Compounds and Chemical BondsZafrel ZaffNo ratings yet
- DOSIMETRYDocument82 pagesDOSIMETRYSimon LucianNo ratings yet
- Nuclear Chemistry - L2Document11 pagesNuclear Chemistry - L2Simon LucianNo ratings yet
- Microbiology AssignmentDocument5 pagesMicrobiology AssignmentSimon LucianNo ratings yet
- Lecture OneDocument25 pagesLecture OneSimon LucianNo ratings yet
- Alkaline MetalsDocument29 pagesAlkaline MetalsAigerim TurlanovaNo ratings yet
- 9th Smart Syllabus 2020Document22 pages9th Smart Syllabus 2020Sana KhanNo ratings yet
- Unit 2 CapsuleDocument104 pagesUnit 2 Capsuleabdullah2020No ratings yet
- Open Newel StaircaseDocument4 pagesOpen Newel StaircaseSujan Dhoj KhadkaNo ratings yet
- DRB - Lecture On Kinetics of Curvilinear TranslationDocument7 pagesDRB - Lecture On Kinetics of Curvilinear TranslationVianca CamilLe Incognito-Castillo PanganibanNo ratings yet
- Mechanics of Materials: A Materials Science PerspectiveDocument7 pagesMechanics of Materials: A Materials Science PerspectiveHarshaNo ratings yet
- Chapter 17 NotesDocument72 pagesChapter 17 Notesapi-30718309No ratings yet
- Viscosity of Neodols PDFDocument3 pagesViscosity of Neodols PDFADITYA MAHANo ratings yet
- B.T.A. Slurry For FlorbacDocument8 pagesB.T.A. Slurry For FlorbacZainudinNo ratings yet
- Produced Water Treatment TechnologiesDocument21 pagesProduced Water Treatment TechnologiesAhmed Ali100% (1)
- Choice of Technique in Gold Assay of JewelleryDocument11 pagesChoice of Technique in Gold Assay of Jewelleryamukti27No ratings yet
- PBA Updated 2024 HSSCDocument12 pagesPBA Updated 2024 HSSCnopeyeah88No ratings yet
- Approval of Dısınfectıon Methods and Equıpment For Aquaculture in NorwayDocument4 pagesApproval of Dısınfectıon Methods and Equıpment For Aquaculture in NorwayFeyza KaragözNo ratings yet
- Survey of The Elementary Principles - Part - 1Document25 pagesSurvey of The Elementary Principles - Part - 1Shakeel Ahmad KasuriNo ratings yet
- 1 Conduct and Uses of Jar Tests: 218 Research and Technology Journal AwwaDocument6 pages1 Conduct and Uses of Jar Tests: 218 Research and Technology Journal AwwaFabian A NeiraNo ratings yet
- Electrical Graphic SymbolDocument5 pagesElectrical Graphic SymbolIslam ShoukryNo ratings yet
- The Interaction Between Any Two Charges Is Completely Unaffected by The Presence of OthersDocument9 pagesThe Interaction Between Any Two Charges Is Completely Unaffected by The Presence of Otherssuma mumuNo ratings yet
- Bingham Reiner EquationDocument6 pagesBingham Reiner EquationSaad Ahmed100% (2)
- Astm C150-C150M-22Document9 pagesAstm C150-C150M-22mustafa97a141No ratings yet
- DCVG MethodDocument12 pagesDCVG MethodAmanSharmaNo ratings yet
- Module 4-Principles of Measurement and Laboratory Mathematics in Clinical ChemistryDocument6 pagesModule 4-Principles of Measurement and Laboratory Mathematics in Clinical ChemistryAllyah Ross DuqueNo ratings yet
- Vedanta - ShortlistDocument18 pagesVedanta - ShortlistStorm ThorNo ratings yet
- Production: Ethers, Aliphatic)Document6 pagesProduction: Ethers, Aliphatic)Harsh ShahNo ratings yet
- Class 10 Math CBSEDocument56 pagesClass 10 Math CBSESonali SinghNo ratings yet
- Examinaton of phsics امتحان في مادة الفيزياء الجامعيةDocument3 pagesExaminaton of phsics امتحان في مادة الفيزياء الجامعيةmaanibrahimNo ratings yet
- Near-Infrared Spectroscopy: TheoryDocument5 pagesNear-Infrared Spectroscopy: TheoryThea RadüntzNo ratings yet
- 6-2 Stark EffectDocument44 pages6-2 Stark Effectcamzmila6No ratings yet