Professional Documents
Culture Documents
prion
prion
Uploaded by
Lilien NagyCopyright:
Available Formats
You might also like
- Rions: Misfolded ProteinDocument28 pagesRions: Misfolded Proteinskurmi0No ratings yet
- Alternative Treatment For Wildlife PDFDocument46 pagesAlternative Treatment For Wildlife PDFPaula Andrea Rodriguez100% (1)
- Packrat 14 PDFDocument86 pagesPackrat 14 PDFkat100% (1)
- Httpsgrado - Ucuenca.edu - Ecpluginfile.php860301mod Foldercontent0L04 202010 20prions 20reassessment20of20the20germ 13Document7 pagesHttpsgrado - Ucuenca.edu - Ecpluginfile.php860301mod Foldercontent0L04 202010 20prions 20reassessment20of20the20germ 13Belen GuambañaqNo ratings yet
- A Guzzi 2013Document15 pagesA Guzzi 2013Yuridia RodríguezNo ratings yet
- Bovine Spongiform-PrionesDocument10 pagesBovine Spongiform-Prionessebastian GarciaNo ratings yet
- Section VIII-H: Prion Diseases: Occupational InfectionsDocument8 pagesSection VIII-H: Prion Diseases: Occupational InfectionsSebastian SalasNo ratings yet
- Assignment On PrionsDocument22 pagesAssignment On PrionsRinta Moon100% (3)
- Fundamentals of Prions and Their Inactivation (Review)Document7 pagesFundamentals of Prions and Their Inactivation (Review)ManuelNo ratings yet
- Prion-Protein-Biology_cellDocument12 pagesPrion-Protein-Biology_cellLilien NagyNo ratings yet
- History of Prions:: Leomill Mendiola MARCH 06, 2017 Bmls 3B PrionsDocument3 pagesHistory of Prions:: Leomill Mendiola MARCH 06, 2017 Bmls 3B Prionsleo100% (1)
- 7 PrionsDocument12 pages7 PrionsIness ChinyimbaNo ratings yet
- 2-蛋白质-朊蛋白-2011-J Intern Med.xDocument14 pages2-蛋白质-朊蛋白-2011-J Intern Med.xjdskydtNo ratings yet
- PrionsDocument28 pagesPrionsPaula PollNo ratings yet
- Prions by Iris LópezDocument12 pagesPrions by Iris LópezMiguel EscarcegaNo ratings yet
- HHS Public Access: Prion DiseasesDocument39 pagesHHS Public Access: Prion DiseasesEstu Kurnia Tiara FianiNo ratings yet
- Prion Diseases: Primary StructuresDocument4 pagesPrion Diseases: Primary StructuresDennis ValdezNo ratings yet
- Human Prion Disease Molecular Pathogenesis and Possible Therapeutic Targets and StrategiesDocument15 pagesHuman Prion Disease Molecular Pathogenesis and Possible Therapeutic Targets and StrategiesMiguel HMNo ratings yet
- PrionDocument22 pagesPrionAnushkaNo ratings yet
- Artigo 14 - Kuru Prions and Sporadic Creutzfeldt-Jakob Disease Prions Have Equivalent Transmission Properties in Transgenic and Wild-Type MiceDocument6 pagesArtigo 14 - Kuru Prions and Sporadic Creutzfeldt-Jakob Disease Prions Have Equivalent Transmission Properties in Transgenic and Wild-Type Micenancy_reisNo ratings yet
- DR Soto-Prion Hypothesis The End of The ControversyDocument8 pagesDR Soto-Prion Hypothesis The End of The ControversyM Angel Conde CarpinteyroNo ratings yet
- Parkinsons Disease in Adults PDF 1837629189061Document37 pagesParkinsons Disease in Adults PDF 1837629189061Rizqan Fahlevvi AkbarNo ratings yet
- Prion Hypothesis: The End of The Controversy?: Claudio SotoDocument8 pagesPrion Hypothesis: The End of The Controversy?: Claudio SotoGabriel LVNo ratings yet
- Seneff Et Al Diseases 2022Document15 pagesSeneff Et Al Diseases 2022Pack AckNo ratings yet
- PRION: Infectious ProteinDocument30 pagesPRION: Infectious Proteindenysoliynyk100% (1)
- 1 s2.0 S009286740500156X MainDocument12 pages1 s2.0 S009286740500156X MainKadir OzcanNo ratings yet
- IFT Transmissible Spongiform EncephalopathiesDocument11 pagesIFT Transmissible Spongiform EncephalopathiesDavidNo ratings yet
- Presentation 8Document25 pagesPresentation 8Ola-Badmus RahdiyatNo ratings yet
- Breakthroughs in Antemortem Diagnosis of Neurodegenerative DiseasesDocument3 pagesBreakthroughs in Antemortem Diagnosis of Neurodegenerative DiseasesVijay PrajapatiNo ratings yet
- (Article) A Potential Role of The Spike Protein in Neurodegenerative DiseasesDocument16 pages(Article) A Potential Role of The Spike Protein in Neurodegenerative DiseasesWalber HenriqueNo ratings yet
- Neuroinflamacion en PrionicasDocument18 pagesNeuroinflamacion en PrionicasAliciaNo ratings yet
- Viruses 14 00630Document14 pagesViruses 14 00630Arika EffiyanaNo ratings yet
- Neurodegenerative Prion Diseases: by Richard A. BessenDocument10 pagesNeurodegenerative Prion Diseases: by Richard A. BessenIgnacio CFNo ratings yet
- Molecular Pathology of Human Prion DiseasesDocument24 pagesMolecular Pathology of Human Prion DiseasesRoxana MariaNo ratings yet
- Prion Diseases: Key PointsDocument4 pagesPrion Diseases: Key PointsD SNo ratings yet
- Prions MPath2013!14!2Document96 pagesPrions MPath2013!14!2Kaye EvangelistaNo ratings yet
- Module 10 - Mycology and VirologyDocument11 pagesModule 10 - Mycology and VirologyKaycee Jane BiñasNo ratings yet
- Prions, Prionoids and Protein Misfolding Disorders Claudia Scheckel (2018)Document14 pagesPrions, Prionoids and Protein Misfolding Disorders Claudia Scheckel (2018)Cambiador de MundoNo ratings yet
- Generalities:: Transmissible Spongiform EncephalopathiesDocument44 pagesGeneralities:: Transmissible Spongiform EncephalopathiesjosephNo ratings yet
- Hipotesis Solo ProteinaDocument14 pagesHipotesis Solo ProteinaAliciaNo ratings yet
- Prion Diseases - Scrapie and Bovine Spongiform EncephalopathyDocument21 pagesPrion Diseases - Scrapie and Bovine Spongiform EncephalopathyELIJAH EUMIR CUNANANNo ratings yet
- Pri OnesDocument1 pagePri OnesJhon NovilloNo ratings yet
- The First Non Prion Pathogen Identified Neurotropic Influenza VirusDocument7 pagesThe First Non Prion Pathogen Identified Neurotropic Influenza VirusRenataNo ratings yet
- PrionDocument6 pagesPrionlajani6258No ratings yet
- Virus: Schematic Diagram of The Hexon of A Virus Capsid Made From Two Protein MoleculeDocument7 pagesVirus: Schematic Diagram of The Hexon of A Virus Capsid Made From Two Protein MoleculeAli RazaNo ratings yet
- 2016 - CNS 6 - CJDDocument34 pages2016 - CNS 6 - CJDAfikah AimanNo ratings yet
- Carrier Macrmoleculas 01Document12 pagesCarrier Macrmoleculas 01Ale MarinNo ratings yet
- Microbial Pathogenesis: SciencedirectDocument11 pagesMicrobial Pathogenesis: SciencedirectFerdinand Prayogo Cahyo SantosoNo ratings yet
- Prion: Maxs U.E. Sanam FKH UndanaDocument8 pagesPrion: Maxs U.E. Sanam FKH UndanaEzza Martya0% (1)
- Slow Virus Diseases: Dr.T.V.Rao MDDocument38 pagesSlow Virus Diseases: Dr.T.V.Rao MDshravaniNo ratings yet
- Propagation of Human SCJD Prions in Organotypic Slice CultureDocument1 pagePropagation of Human SCJD Prions in Organotypic Slice CultureVera SouzaNo ratings yet
- Prion OverviewDocument9 pagesPrion Overviewapi-196020598No ratings yet
- Pneumocystis: PneumoniaDocument13 pagesPneumocystis: PneumoniazikryauliaNo ratings yet
- A Review On Prions: Pharmaceutical Microbiology Tujan LiddawiehDocument22 pagesA Review On Prions: Pharmaceutical Microbiology Tujan LiddawiehMayson BaliNo ratings yet
- Genetic Counseling For Prion Disease Updates and Best - 2022 - Genetics in MediDocument11 pagesGenetic Counseling For Prion Disease Updates and Best - 2022 - Genetics in Medironaldquezada038No ratings yet
- Plasmodium Knowlesi in Human, Indonesian BorneoDocument3 pagesPlasmodium Knowlesi in Human, Indonesian BorneoDanny. JayNo ratings yet
- Infectious Diseases General 21Document151 pagesInfectious Diseases General 21Esma GündoğduNo ratings yet
- Sem4 Prion VGDocument21 pagesSem4 Prion VGKaye EvangelistaNo ratings yet
- Plasmodium KnowlesiDocument7 pagesPlasmodium KnowlesiAnantaBenvenutoNo ratings yet
- Viroids and PrionsDocument14 pagesViroids and PrionsKavisa Ghosh100% (1)
- The Pathological Protein: Mad Cow, Chronic Wasting, and Other Deadly Prion DiseasesFrom EverandThe Pathological Protein: Mad Cow, Chronic Wasting, and Other Deadly Prion DiseasesRating: 4 out of 5 stars4/5 (6)
- The Infection Game Supplement: new infections, retroviruses and pandemicsFrom EverandThe Infection Game Supplement: new infections, retroviruses and pandemicsNo ratings yet
- Polycombing-the-Genome--PcG,-trxG,-and-Chromatin-SDocument4 pagesPolycombing-the-Genome--PcG,-trxG,-and-Chromatin-SLilien NagyNo ratings yet
- Odorant-Receptor-Localization-to-Olfactory-Cilia-IDocument12 pagesOdorant-Receptor-Localization-to-Olfactory-Cilia-ILilien NagyNo ratings yet
- TIP47--A-Cargo-Selection-Device-for-Mannose-6-PhosDocument11 pagesTIP47--A-Cargo-Selection-Device-for-Mannose-6-PhosLilien NagyNo ratings yet
- Nibrin,-a-Novel-DNA-Double-Strand-Break-Repair-ProDocument10 pagesNibrin,-a-Novel-DNA-Double-Strand-Break-Repair-ProLilien NagyNo ratings yet
- Imprinting-and-the-Initiation-of-Gene-Silencing-inDocument4 pagesImprinting-and-the-Initiation-of-Gene-Silencing-inLilien NagyNo ratings yet
- The-hMre11-hRad50-Protein-Complex-and-Nijmegen-BreDocument10 pagesThe-hMre11-hRad50-Protein-Complex-and-Nijmegen-BreLilien NagyNo ratings yet
- Hospital Waste ManagementDocument40 pagesHospital Waste Managementamir khanNo ratings yet
- Essential Health Services Package of Ethiopia 2019Document156 pagesEssential Health Services Package of Ethiopia 2019joseph100% (1)
- Barium Enema and Biliary System1Document18 pagesBarium Enema and Biliary System1sheenamarielaruyaNo ratings yet
- LP CKDDocument26 pagesLP CKDrissaalhuznaNo ratings yet
- An Evaluation of SymptomsDocument8 pagesAn Evaluation of SymptomsHomeoDr Babar AminNo ratings yet
- EPISTAXISDocument2 pagesEPISTAXISRUTH RosilinNo ratings yet
- Case Study On Ulcerative ColitisDocument19 pagesCase Study On Ulcerative Colitisabirami pNo ratings yet
- The Gluten Connection - Walter VeithDocument5 pagesThe Gluten Connection - Walter VeithGabriela Rojas FusNo ratings yet
- Trauma Sensitive Yoga - 14 Precautions To Keep in Mind When Teaching - Tummee - Com Trusted by Yoga TherapistsDocument5 pagesTrauma Sensitive Yoga - 14 Precautions To Keep in Mind When Teaching - Tummee - Com Trusted by Yoga TherapistspuNo ratings yet
- Ohio HOSA Maya Rao VTK Research Poster 21-22Document1 pageOhio HOSA Maya Rao VTK Research Poster 21-22Maya RaoNo ratings yet
- Bloating and Functional Gastro-Intestinal DisorderDocument15 pagesBloating and Functional Gastro-Intestinal DisorderJayantiNo ratings yet
- Oral Medicine (PDFDrive)Document166 pagesOral Medicine (PDFDrive)Tasha FarahNo ratings yet
- Chapter 5Document9 pagesChapter 5Mae CanlasNo ratings yet
- Jeyakumar Dhileeban Rrroll 41Document34 pagesJeyakumar Dhileeban Rrroll 41Rupesh TamizhaNo ratings yet
- Subsection 2: Warranty Against Hidden Defects of or Encumbrances Upon The Thing Sold (Part 2)Document11 pagesSubsection 2: Warranty Against Hidden Defects of or Encumbrances Upon The Thing Sold (Part 2)WenjunNo ratings yet
- National Consumer Disputes Redressal Commission New Delhi Consumer Case No. 173 of 2011Document7 pagesNational Consumer Disputes Redressal Commission New Delhi Consumer Case No. 173 of 2011Arnav JoshiNo ratings yet
- Manual For The Treatment of Poultry DiseaseDocument75 pagesManual For The Treatment of Poultry DiseaseAzwar ChishtiNo ratings yet
- Hindreances of Having Business Amidst The Pandemic in Marketview Lucena CityDocument8 pagesHindreances of Having Business Amidst The Pandemic in Marketview Lucena CityG E R L I ENo ratings yet
- Poster Schedule Midaoicon 2023Document3 pagesPoster Schedule Midaoicon 2023hiteshtakNo ratings yet
- MCQ's in Psychiatry & Psychology Anil Kakunje Ips QuizDocument211 pagesMCQ's in Psychiatry & Psychology Anil Kakunje Ips QuizAbhishek Kumar SharmaNo ratings yet
- Breast Complications Content PDFDocument4 pagesBreast Complications Content PDFSrija GhoshNo ratings yet
- CVID-19 Vaccine IngredientsDocument25 pagesCVID-19 Vaccine IngredientsFloppyNo ratings yet
- Team Code XXXIV (PETITIONER)Document22 pagesTeam Code XXXIV (PETITIONER)PRASHANTNo ratings yet
- NOTES CD Lecture Generic 2022Document182 pagesNOTES CD Lecture Generic 2022Meryville JacildoNo ratings yet
- Vol5 Issue4 02 PDFDocument3 pagesVol5 Issue4 02 PDFRyan RachmawanNo ratings yet
- Grenada Weekly Surveillance Bulletin (Ew 25)Document4 pagesGrenada Weekly Surveillance Bulletin (Ew 25)Denis BainNo ratings yet
- A. Facial Mask B. Non-Rebreather Mask C. Venturi MaskDocument17 pagesA. Facial Mask B. Non-Rebreather Mask C. Venturi Maskshivani kaushalNo ratings yet
- MumpsDocument16 pagesMumpsRahul DhakerNo ratings yet
prion
prion
Uploaded by
Lilien NagyCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
prion
prion
Uploaded by
Lilien NagyCopyright:
Available Formats
Transmissible Spongiform Encephalopathy
Prion Protein Diseases
Lisa Kennedy, Dylan Bradford, Madi Hoagland Henefield, Anders Ohman
Advisor: Dr. Todd Livdahl
Background Molecular Biology of the
Transmission Routes Prion diseases (formally, Transmissible Spongiform Encephalopathies) are neurodegenerative conditions in mammals that are Pathogenesis of TSE
It is commonly held that prion diseases are mainly transmitted hypothesized to be caused by an infectious protein agent without the involvement of nucleic information or additional transport vectors. The
between animals via ingestion or orally. A number of ingestion routes have theorized prion agent is a modified form of the cell membrane protein PrP C, which is misfolded into the pathogenic form of the protein,
been identified that cause disease in humans as well as other animals Prion disease are unique in that they are
which is referred to as PrPSc. These prion proteins are able to convert normal protein molecules into the prion form, and the proteins are
including deer, elk, and cattle. extremely resistant to denaturation by temperature or chemical agents, making them both difficult to destroy once in a host as well as transmissible particles that lack any form of nucleic acid,
The most common and surest way is to ingest tissue from an capable of surviving in an exposed environment for an extended period of time. and yet are still infectious (Prusiner, 1998). While prion
infected animal, the brain tissue having the highest concentration of Prion diseases have been documented for decades (centuries, in the case of Scrapie), but the discovery of the prion protein itself was diseases are not completely understood, it is clear the
dangerous prions. Omnivorous and carnivorous animals are susceptible to made as recently as 1997 by Stanley B. Prusiner, who won a Nobel Prize for his work. The mechanics of the disease are still largely disease is caused by an accumulation in the brain of a
this mode of infection, and exhibits a very real danger to human hunters of unknown. They can affect many lineages of mammals, from rodents to ruminants, as well as humans. Prion disease in livestock has serious misfolded cellular protein known as the prion protein
deer and elk. A study of humans that were exposed to Chronic Wasting implications for human food source populations, as well as for potential infection in humans, as the Bovine Spongiform Encephalopathy
Disease (a closely related prion disease) via hunted deer at a wild game (PrPc) (Campana et al., 2008).
may potentially be linked to a variant of Creutzfeldt-Jakob Disease in humans. There is no current cure, inoculation, or treatment for prion
feast suggested two levels of risk, exposure and ingestion (Garruto et al. diseases, and livestock protection strategies largely include quarantine and destruction of infected organisms. The normally occurring prion protein is converted
2008). Those subject that had actually ingested venison that could have into the modified infectious protein PrPSc
come from an infected deer were said to be of high-risk and are currently Symptoms: posttranslationally. During this process PrPc misfolds
under close watch in case CWD is found to transmit between deer and TSEs cause the formation of holes in the host nervous tissue, causing a sponge-like appearance from which the term ‘spongiform’ is into PrPSc which contains a high number of β-sheets. The
humans. derived. Damage to the nervous tissue causes a number of deteriorative cognitive and behavioral conditions in prion hosts of all susceptible process during which the normal protein changes to the
For herbivores it was found that prions, in the form of Scrapie Agent species, typically including dementia, acute mood changes, and issues with physical coordination and control.
263k, are able to stay dormant in soil for at least as long as 29 months and abnormal takes place when some of the α-helices and
cause infection (Seidel et al. 2007). The study was done using Syrian coils of its structure refold into β-sheets. This structural
hamsters and PrPSC were found to infect and proliferate within the hamsters alteration causes less understood but still profound
when fed soil samples. Aqueous extract was also shown to induce disease Modeling the System changes in the chemical properties of the PrP. The
in the reporter animals. These findings suggest a highly dangerous scenario changes that result in the PrPSc have been shown to act
for cattle and sheep flocks. Prions can be deposited by decaying animals, as The model below calculates the number of new cases of as a template upon which PrPc is refolded into PrPSc
well as infected organs including placenta, amniotic fluid, and possibly Transmission Rate
BSE that result from the use of infected BSE cattle in cattle
urine (Gregori et al. 2008), making grazing animals highly susceptible. Cattle population (Prusiner, 1998). This is how the misfolded protein is
feed. The practice of “recycling” cattle was relatively able to become infectious to other prion proteins around
There still remains much research to be done on prion diseases as a
whole including transmission routes. The way they are passed between BSE Infecctions BSE Cattle
common before the outbreaks of BSE in the UK. This it. Furthermore, PrPSc has shown the ability to resist
animals is very important to determine if only to protect humans from STELLA model (fig. 1) utilizes data used in a published degradation even after the death of its host for up to
possible cross-species infection. Given the amount of red meat consumed model used to calculate the hypothetical reproductive ratio months or even years at a time, and still be infectious to a
in the United States, a large portion of infected but undetected meat due to a of BSE, as well as actual data on the cattle population of
Proportion Infectivity new host if picked up.
lack of knowledge could cause a large loss of life. the UK in 1986.
Types of TSE Infectivity Passed
Material with BSE Infectivity
Prion disease occurs in a number of different mammals, and strains of prion
disease typically only affect peers in the species and closely-related lineages
(Prusiner, S.B. et al 1991). There are three primary categories of prion diseases:
Sporadic, where the cause is unknown ; Familial, where the disease is caused by the
Cattle p o p ulation 12. 7 million*
Reduction Rate
genetics of the host; and Acquired, which is transmissible between organisms, either of Infectvivity Trans mission rate .001
directly or through the environment.
Pr op or tio n infe ctivity .8 5
New Cases of BSE from MBM
There are four primary prion diseases that affect humans: Creutzfeldt-Jakob Disease,
Kuru, Fatal Familial Insomnia, and Gerstmann-Straussler-Scheinker Syndrome. CJD BSE infectivity in MBM
Red uctio n rate of infectivity .1
has variant strains in all three categories, while Kuru is an Acquired disease and both
FFI and GSSS are Familial (Soldevila, M. et al 2006). Symptoms of CJD, the most Cattle Feed with Pr op or tio n of MBM in Cattl e .2
common human prion disease with 1-2 instances in a million people annually, has the
Infected MBM
Redction of BSE Material Fe e d
symptoms of dementia, issues with coordination, and visual hallucinations. GSSS
Proportion of MBM
New cas e s o f BSE 34
3722
shares the symptoms of dementia and coordination problems, but also results in in Cattle Feed
difficulty speaking. Sufferers of Kuru have laughing fits and trembling fits. FFI
Graph 1
*so urc e : BSEinq uiry.g o v.uk
causes insomnia, hallucinations, and also dementia (Monari, L. et al 1994).
Deer, Elks, and Moose are affected by Chronic Wasting Disease, an Acquired
condition that is transmissible directly or through contact with a shared environment
(Joly, D.O. et al 2003). CWD was first contained to the Central United States, but has
since spread to multiple areas of the US and Canada, including game parks. It causes
changes in host mood, diet, and behavior. 1947: 1967: Chronic
1921: Transmissible Wasting 1981: CWD 1996: Variant
Sheep and Goats are affected by Scrapie, the oldest-known prion disease first named
in 1732 (www.defra.gov.uk). Scrapie affects both the nervous system as well as the
Creutzfeldt-Jakob mink Disease CWD: wild Creutzfeldt-
tonsils and the distal ileum, and causes behavioral abnormalities such as the disease (CJD) encephalopathy captivity populations Jakob Disease
compulsive scraping of the host on objects in the environment from which the
diseases’ name is derived. Although found in Europe and in America, successful
quarantine has kept the disease out of Oceanian populations (Parsonson, I.M. 1996). TIMELINE
Cows are affected by Bovine Spongiform Encephalopathy. While BSE (a.k.a. Mad
Cow Disease) is a strictly Familial disease, domestic animal practices for feeding Kuru:
livestock resulted in direct consumption of infected brain tissue as animal feed, and 1732*: 1936: Gerstmann 1974: Fatal 1986: 1997: Prion 2003: Feline
therefore spread the disease from individual to individual (Prusiner, S.B. 1997). BSE Scrapie Straussler-Scheinker 1959 Familial BSE protein spongiform
leads to the formation of protein fibers in the brain, leading to the presence of holes in Syndrome (GSSS) Insomnia discovered*** encephalopathy
the tissue. Symptoms include nervous or aggressive behavior, weight loss, and
coordination problems, but can take years to actually appear. *Timeline not to scale
**The dates indicate descriptions and identifications of the respective diseases.
At the earlier dates, cause of disease by the prion protein was not known
***By Stanley B. Prusiner winner of the Nobel Prize in Physiology or
Medicine 1997
You might also like
- Rions: Misfolded ProteinDocument28 pagesRions: Misfolded Proteinskurmi0No ratings yet
- Alternative Treatment For Wildlife PDFDocument46 pagesAlternative Treatment For Wildlife PDFPaula Andrea Rodriguez100% (1)
- Packrat 14 PDFDocument86 pagesPackrat 14 PDFkat100% (1)
- Httpsgrado - Ucuenca.edu - Ecpluginfile.php860301mod Foldercontent0L04 202010 20prions 20reassessment20of20the20germ 13Document7 pagesHttpsgrado - Ucuenca.edu - Ecpluginfile.php860301mod Foldercontent0L04 202010 20prions 20reassessment20of20the20germ 13Belen GuambañaqNo ratings yet
- A Guzzi 2013Document15 pagesA Guzzi 2013Yuridia RodríguezNo ratings yet
- Bovine Spongiform-PrionesDocument10 pagesBovine Spongiform-Prionessebastian GarciaNo ratings yet
- Section VIII-H: Prion Diseases: Occupational InfectionsDocument8 pagesSection VIII-H: Prion Diseases: Occupational InfectionsSebastian SalasNo ratings yet
- Assignment On PrionsDocument22 pagesAssignment On PrionsRinta Moon100% (3)
- Fundamentals of Prions and Their Inactivation (Review)Document7 pagesFundamentals of Prions and Their Inactivation (Review)ManuelNo ratings yet
- Prion-Protein-Biology_cellDocument12 pagesPrion-Protein-Biology_cellLilien NagyNo ratings yet
- History of Prions:: Leomill Mendiola MARCH 06, 2017 Bmls 3B PrionsDocument3 pagesHistory of Prions:: Leomill Mendiola MARCH 06, 2017 Bmls 3B Prionsleo100% (1)
- 7 PrionsDocument12 pages7 PrionsIness ChinyimbaNo ratings yet
- 2-蛋白质-朊蛋白-2011-J Intern Med.xDocument14 pages2-蛋白质-朊蛋白-2011-J Intern Med.xjdskydtNo ratings yet
- PrionsDocument28 pagesPrionsPaula PollNo ratings yet
- Prions by Iris LópezDocument12 pagesPrions by Iris LópezMiguel EscarcegaNo ratings yet
- HHS Public Access: Prion DiseasesDocument39 pagesHHS Public Access: Prion DiseasesEstu Kurnia Tiara FianiNo ratings yet
- Prion Diseases: Primary StructuresDocument4 pagesPrion Diseases: Primary StructuresDennis ValdezNo ratings yet
- Human Prion Disease Molecular Pathogenesis and Possible Therapeutic Targets and StrategiesDocument15 pagesHuman Prion Disease Molecular Pathogenesis and Possible Therapeutic Targets and StrategiesMiguel HMNo ratings yet
- PrionDocument22 pagesPrionAnushkaNo ratings yet
- Artigo 14 - Kuru Prions and Sporadic Creutzfeldt-Jakob Disease Prions Have Equivalent Transmission Properties in Transgenic and Wild-Type MiceDocument6 pagesArtigo 14 - Kuru Prions and Sporadic Creutzfeldt-Jakob Disease Prions Have Equivalent Transmission Properties in Transgenic and Wild-Type Micenancy_reisNo ratings yet
- DR Soto-Prion Hypothesis The End of The ControversyDocument8 pagesDR Soto-Prion Hypothesis The End of The ControversyM Angel Conde CarpinteyroNo ratings yet
- Parkinsons Disease in Adults PDF 1837629189061Document37 pagesParkinsons Disease in Adults PDF 1837629189061Rizqan Fahlevvi AkbarNo ratings yet
- Prion Hypothesis: The End of The Controversy?: Claudio SotoDocument8 pagesPrion Hypothesis: The End of The Controversy?: Claudio SotoGabriel LVNo ratings yet
- Seneff Et Al Diseases 2022Document15 pagesSeneff Et Al Diseases 2022Pack AckNo ratings yet
- PRION: Infectious ProteinDocument30 pagesPRION: Infectious Proteindenysoliynyk100% (1)
- 1 s2.0 S009286740500156X MainDocument12 pages1 s2.0 S009286740500156X MainKadir OzcanNo ratings yet
- IFT Transmissible Spongiform EncephalopathiesDocument11 pagesIFT Transmissible Spongiform EncephalopathiesDavidNo ratings yet
- Presentation 8Document25 pagesPresentation 8Ola-Badmus RahdiyatNo ratings yet
- Breakthroughs in Antemortem Diagnosis of Neurodegenerative DiseasesDocument3 pagesBreakthroughs in Antemortem Diagnosis of Neurodegenerative DiseasesVijay PrajapatiNo ratings yet
- (Article) A Potential Role of The Spike Protein in Neurodegenerative DiseasesDocument16 pages(Article) A Potential Role of The Spike Protein in Neurodegenerative DiseasesWalber HenriqueNo ratings yet
- Neuroinflamacion en PrionicasDocument18 pagesNeuroinflamacion en PrionicasAliciaNo ratings yet
- Viruses 14 00630Document14 pagesViruses 14 00630Arika EffiyanaNo ratings yet
- Neurodegenerative Prion Diseases: by Richard A. BessenDocument10 pagesNeurodegenerative Prion Diseases: by Richard A. BessenIgnacio CFNo ratings yet
- Molecular Pathology of Human Prion DiseasesDocument24 pagesMolecular Pathology of Human Prion DiseasesRoxana MariaNo ratings yet
- Prion Diseases: Key PointsDocument4 pagesPrion Diseases: Key PointsD SNo ratings yet
- Prions MPath2013!14!2Document96 pagesPrions MPath2013!14!2Kaye EvangelistaNo ratings yet
- Module 10 - Mycology and VirologyDocument11 pagesModule 10 - Mycology and VirologyKaycee Jane BiñasNo ratings yet
- Prions, Prionoids and Protein Misfolding Disorders Claudia Scheckel (2018)Document14 pagesPrions, Prionoids and Protein Misfolding Disorders Claudia Scheckel (2018)Cambiador de MundoNo ratings yet
- Generalities:: Transmissible Spongiform EncephalopathiesDocument44 pagesGeneralities:: Transmissible Spongiform EncephalopathiesjosephNo ratings yet
- Hipotesis Solo ProteinaDocument14 pagesHipotesis Solo ProteinaAliciaNo ratings yet
- Prion Diseases - Scrapie and Bovine Spongiform EncephalopathyDocument21 pagesPrion Diseases - Scrapie and Bovine Spongiform EncephalopathyELIJAH EUMIR CUNANANNo ratings yet
- Pri OnesDocument1 pagePri OnesJhon NovilloNo ratings yet
- The First Non Prion Pathogen Identified Neurotropic Influenza VirusDocument7 pagesThe First Non Prion Pathogen Identified Neurotropic Influenza VirusRenataNo ratings yet
- PrionDocument6 pagesPrionlajani6258No ratings yet
- Virus: Schematic Diagram of The Hexon of A Virus Capsid Made From Two Protein MoleculeDocument7 pagesVirus: Schematic Diagram of The Hexon of A Virus Capsid Made From Two Protein MoleculeAli RazaNo ratings yet
- 2016 - CNS 6 - CJDDocument34 pages2016 - CNS 6 - CJDAfikah AimanNo ratings yet
- Carrier Macrmoleculas 01Document12 pagesCarrier Macrmoleculas 01Ale MarinNo ratings yet
- Microbial Pathogenesis: SciencedirectDocument11 pagesMicrobial Pathogenesis: SciencedirectFerdinand Prayogo Cahyo SantosoNo ratings yet
- Prion: Maxs U.E. Sanam FKH UndanaDocument8 pagesPrion: Maxs U.E. Sanam FKH UndanaEzza Martya0% (1)
- Slow Virus Diseases: Dr.T.V.Rao MDDocument38 pagesSlow Virus Diseases: Dr.T.V.Rao MDshravaniNo ratings yet
- Propagation of Human SCJD Prions in Organotypic Slice CultureDocument1 pagePropagation of Human SCJD Prions in Organotypic Slice CultureVera SouzaNo ratings yet
- Prion OverviewDocument9 pagesPrion Overviewapi-196020598No ratings yet
- Pneumocystis: PneumoniaDocument13 pagesPneumocystis: PneumoniazikryauliaNo ratings yet
- A Review On Prions: Pharmaceutical Microbiology Tujan LiddawiehDocument22 pagesA Review On Prions: Pharmaceutical Microbiology Tujan LiddawiehMayson BaliNo ratings yet
- Genetic Counseling For Prion Disease Updates and Best - 2022 - Genetics in MediDocument11 pagesGenetic Counseling For Prion Disease Updates and Best - 2022 - Genetics in Medironaldquezada038No ratings yet
- Plasmodium Knowlesi in Human, Indonesian BorneoDocument3 pagesPlasmodium Knowlesi in Human, Indonesian BorneoDanny. JayNo ratings yet
- Infectious Diseases General 21Document151 pagesInfectious Diseases General 21Esma GündoğduNo ratings yet
- Sem4 Prion VGDocument21 pagesSem4 Prion VGKaye EvangelistaNo ratings yet
- Plasmodium KnowlesiDocument7 pagesPlasmodium KnowlesiAnantaBenvenutoNo ratings yet
- Viroids and PrionsDocument14 pagesViroids and PrionsKavisa Ghosh100% (1)
- The Pathological Protein: Mad Cow, Chronic Wasting, and Other Deadly Prion DiseasesFrom EverandThe Pathological Protein: Mad Cow, Chronic Wasting, and Other Deadly Prion DiseasesRating: 4 out of 5 stars4/5 (6)
- The Infection Game Supplement: new infections, retroviruses and pandemicsFrom EverandThe Infection Game Supplement: new infections, retroviruses and pandemicsNo ratings yet
- Polycombing-the-Genome--PcG,-trxG,-and-Chromatin-SDocument4 pagesPolycombing-the-Genome--PcG,-trxG,-and-Chromatin-SLilien NagyNo ratings yet
- Odorant-Receptor-Localization-to-Olfactory-Cilia-IDocument12 pagesOdorant-Receptor-Localization-to-Olfactory-Cilia-ILilien NagyNo ratings yet
- TIP47--A-Cargo-Selection-Device-for-Mannose-6-PhosDocument11 pagesTIP47--A-Cargo-Selection-Device-for-Mannose-6-PhosLilien NagyNo ratings yet
- Nibrin,-a-Novel-DNA-Double-Strand-Break-Repair-ProDocument10 pagesNibrin,-a-Novel-DNA-Double-Strand-Break-Repair-ProLilien NagyNo ratings yet
- Imprinting-and-the-Initiation-of-Gene-Silencing-inDocument4 pagesImprinting-and-the-Initiation-of-Gene-Silencing-inLilien NagyNo ratings yet
- The-hMre11-hRad50-Protein-Complex-and-Nijmegen-BreDocument10 pagesThe-hMre11-hRad50-Protein-Complex-and-Nijmegen-BreLilien NagyNo ratings yet
- Hospital Waste ManagementDocument40 pagesHospital Waste Managementamir khanNo ratings yet
- Essential Health Services Package of Ethiopia 2019Document156 pagesEssential Health Services Package of Ethiopia 2019joseph100% (1)
- Barium Enema and Biliary System1Document18 pagesBarium Enema and Biliary System1sheenamarielaruyaNo ratings yet
- LP CKDDocument26 pagesLP CKDrissaalhuznaNo ratings yet
- An Evaluation of SymptomsDocument8 pagesAn Evaluation of SymptomsHomeoDr Babar AminNo ratings yet
- EPISTAXISDocument2 pagesEPISTAXISRUTH RosilinNo ratings yet
- Case Study On Ulcerative ColitisDocument19 pagesCase Study On Ulcerative Colitisabirami pNo ratings yet
- The Gluten Connection - Walter VeithDocument5 pagesThe Gluten Connection - Walter VeithGabriela Rojas FusNo ratings yet
- Trauma Sensitive Yoga - 14 Precautions To Keep in Mind When Teaching - Tummee - Com Trusted by Yoga TherapistsDocument5 pagesTrauma Sensitive Yoga - 14 Precautions To Keep in Mind When Teaching - Tummee - Com Trusted by Yoga TherapistspuNo ratings yet
- Ohio HOSA Maya Rao VTK Research Poster 21-22Document1 pageOhio HOSA Maya Rao VTK Research Poster 21-22Maya RaoNo ratings yet
- Bloating and Functional Gastro-Intestinal DisorderDocument15 pagesBloating and Functional Gastro-Intestinal DisorderJayantiNo ratings yet
- Oral Medicine (PDFDrive)Document166 pagesOral Medicine (PDFDrive)Tasha FarahNo ratings yet
- Chapter 5Document9 pagesChapter 5Mae CanlasNo ratings yet
- Jeyakumar Dhileeban Rrroll 41Document34 pagesJeyakumar Dhileeban Rrroll 41Rupesh TamizhaNo ratings yet
- Subsection 2: Warranty Against Hidden Defects of or Encumbrances Upon The Thing Sold (Part 2)Document11 pagesSubsection 2: Warranty Against Hidden Defects of or Encumbrances Upon The Thing Sold (Part 2)WenjunNo ratings yet
- National Consumer Disputes Redressal Commission New Delhi Consumer Case No. 173 of 2011Document7 pagesNational Consumer Disputes Redressal Commission New Delhi Consumer Case No. 173 of 2011Arnav JoshiNo ratings yet
- Manual For The Treatment of Poultry DiseaseDocument75 pagesManual For The Treatment of Poultry DiseaseAzwar ChishtiNo ratings yet
- Hindreances of Having Business Amidst The Pandemic in Marketview Lucena CityDocument8 pagesHindreances of Having Business Amidst The Pandemic in Marketview Lucena CityG E R L I ENo ratings yet
- Poster Schedule Midaoicon 2023Document3 pagesPoster Schedule Midaoicon 2023hiteshtakNo ratings yet
- MCQ's in Psychiatry & Psychology Anil Kakunje Ips QuizDocument211 pagesMCQ's in Psychiatry & Psychology Anil Kakunje Ips QuizAbhishek Kumar SharmaNo ratings yet
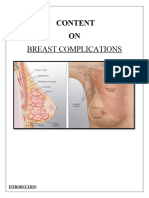
- Breast Complications Content PDFDocument4 pagesBreast Complications Content PDFSrija GhoshNo ratings yet
- CVID-19 Vaccine IngredientsDocument25 pagesCVID-19 Vaccine IngredientsFloppyNo ratings yet
- Team Code XXXIV (PETITIONER)Document22 pagesTeam Code XXXIV (PETITIONER)PRASHANTNo ratings yet
- NOTES CD Lecture Generic 2022Document182 pagesNOTES CD Lecture Generic 2022Meryville JacildoNo ratings yet
- Vol5 Issue4 02 PDFDocument3 pagesVol5 Issue4 02 PDFRyan RachmawanNo ratings yet
- Grenada Weekly Surveillance Bulletin (Ew 25)Document4 pagesGrenada Weekly Surveillance Bulletin (Ew 25)Denis BainNo ratings yet
- A. Facial Mask B. Non-Rebreather Mask C. Venturi MaskDocument17 pagesA. Facial Mask B. Non-Rebreather Mask C. Venturi Maskshivani kaushalNo ratings yet
- MumpsDocument16 pagesMumpsRahul DhakerNo ratings yet